Abstract
Objective
This study was deigned to evaluate the technique and clinical efficacy of the use of percutaneous transportal sclerotherapy with N-butyl-2-cyanoacrylate (NBCA) for patients with gastric varices.
Materials and Methods
Seven patients were treated by transportal sclerotherapy with the use of NBCA. For transportal sclerotherapy, portal vein catheterization was performed with a 6-Fr sheath by the transhepatic approach. A 5-Fr catheter was introduced into the afferent gastric vein and a microcatheter was advanced through the 5-Fr catheter into the varices. NBCA was injected through the microcatheter in the varices by use of the continuous single-column injection technique. After the procedure, postcontrast computed tomography (CT) was performed on the next day and then every six months. Gastroendoscopy was performed at one week, three months, and then every six months after the procedure.
Results
The technical success rate of the procedure was 88%. In six patients, gastric varices were successfully obliterated with 1-8 mL (mean, 5.4 mL) of a NBCA-Lipiodol mixture injected via a microcatheter. No complications related to the procedure were encountered. As seen on the follow-up endoscopy and CT imaging performed after six months, the presence of gastric varcies was not seen in any of the patients after treatment with the NBCA-Lipiodol mixture and the use of microcoils. Recurrence of gastric varices was not observed during the follow-up period. Worsening of esophageal varices occurred in four patients after transportal sclerotherapy. The serum albumin level increased, the ammonia level decreased and the prothrombin time increased at six months after the procedure (p < 0.05).
Gastric fundal varices are less common than esophageal varices. Esophageal varices occur in approximately 20% of patients with portal hypertension (1). However, patients with bleeding from gastric fundal varices have a poor prognosis and have more severe blood loss, a higher rebleeding rate and a higher mortality rate (1-3). Endoscopic treatment is an alternative for the management of gastric varices with hemorrhage and includes the use of endoscopic injection of sclerosants or thrombin, endoscopic band ligation and the use of other agents (4, 5). However, gastric fundal varices cannot be treated effectively with endoscopic injection sclerotherapy due to systemic embolization and incomplete embolization by the rapid loss of the sclerosing agent because of fast blood flow (1, 6-9).
A transjugular intrahepatic portosystemic shunt (TIPS) has been used for the treatment of gastric fundal varices. The use of a TIPS is less invasive than the use of a surgical shunt, and the TIPS mortality rate (1%) is lower than the mortality rate for the use of surgical shunts (3-15%) (10, 11). However, the use of a TIPS does not always result in regression of gastric fundal varices (12) and its use has not been recommended in patients with hepatic encephalopathy (13, 14).
Recently, balloon-occluded retrograde transvenous obliteration (BRTO) through a gastrorenal shunt has been recognized as a safe and effective treatment for gastric varices (15-23). However, some cases of gastric varices without catheterizable main draining veins cannot be treated by the use of BRTO. Percutaneous transportal sclerotherapy has been recently introduced for the treatment of gastric varices without gastrorenal shunts (24), and satisfactory treatment results have been reported for patients. The present study was performed to evaluate the clinical efficacy and technique associated with the use of percutaneous transportal sclerotherapy of gastric varices without a gastrorenal shunt.
Between January 2005 and March 2007, 31 patients with gastric varices who provided written informed consent were referred to our department for treatment with BRTO. Of these patients, 24 underwent the BRTO procedure through a gastrorenal shunt. Seven patients (six men and one woman; age range: 50-69 years; mean age, 57 years) underwent percutaneous transportal sclerotherapy. Of these patients, six patients did not have catheterizable draining veins through a systemic vein and one patient received percutaneous transhepatic sclerotherapy after failure of BRTO due to rupture of the gastrorenal shunt. The clinical features of the seven patients are summarized in Table 1. The underlying hepatic pathologies were cirrhosis caused by hepatitis B virus in four patients, cirrhosis caused by hepatitis C virus in one patient and alcoholic cirrhosis in two patients. As seen on endoscopy, six patients had active bleeding (spurting or oozing) from the gastric fundal varices or showed signs of recent bleeding, such as a clot or fibrin, and had esophageal varices.
All of the patients were given intramuscular administration of atropine sulfate. All of the patients received local anesthesia that consisted of an intramuscular injection of lidocaine (Jeil Pharm, Taegu, Korea) as well as intravenous sedation (Demerol; Keukdong Pharm, Seoul, Korea). Ultrasonography (US) of the liver was performed to determine the best access route into the portal venous system. Under sterile conditions, access into the portal venous system was gained under US or fluoroscopic guidance, or both. The procedure was always performed under fluoroscopy. Percutaneous transhepatic puncture of the right intrahepatic porto-hepatic vein was performed using a 21-gauge Chiba needle (Cook, Bloomington, IN) under ultrasonographic and fluoroscopic guidance. The needle was exchanged for a 4-Fr coaxial dilator and a 6-Fr sheath (Cook) that were placed over either a 0.018-inch guide wire (Cook) or a 0.035-inch angled hydrophilic guide wire (Terumo, Tokyo, Japan). The 0.035-inch guide wire and a 5-Fr cobra catheter (Cook) were used to traverse the gastric varices. Splenoportography was performed with a 5-Fr catheter to evaluate the afferent gastric veins and drainage veins. The main afferent gastric vein was selected with a 5-Fr catheter and venography was performed to access the varices. In four patients, a microcatheter was advanced through the varices into the drainage vein, and microcoils (Cook) were placed into the drainage vein for reduction of venous flow. In five patients, a microcatheter was advanced into the afferent vein and microcoils were placed into the afferent vein to prevent recanalization. In all patients, a 25% mixture of N-butyl-2-cyanoacrylate, (NBCA) and Lipiodol was injected through the microcatheter with its tip in the varices by use of a continuous single-column injection technique until all of the varices were opacified with the mixture. The microcatheter
After the procedure, postcontrast computed tomography (CT) was performed on the next day and then every six months. Gastroendoscopy was performed at one week, three months and then every six months after the procedure. In addition, hepatic function and blood cell counts were monitored during the follow-up period. A plain chest PA X-ray was obtained for each day during the hospital stay to detect a distal embolization of the pulmonary artery.
Technical success was defined as complete clotting of the gastric varices as observed on CT images in the portal phase on the day following treatment. Endoscopic and CT findings of gastric varices, hepatic function tests including measurement of the levels of serum albumin, bilirubin, ammonia, prothrombin time and the presence of symptoms were evaluated after the treatment. Significant differences in the hepatic function tests before and after treatment were determined by use of the Student's t test. A p value of less than 0.05 was considered as statistically significant.
Percutaneous transportal sclerotherapy with the use of an NBCA-Lipiodol mixture was technically successful in six patients (85.7%), including in one patient where the procedure was performed after failure of BRTO. The technical results are shown in Table 2. The number of obliterations of the afferent vein was one in three patients, two in two patients and three in two patients. The volume of the injected NBCA-Lipiodol mixture ranged from 1 to 8 mL (mean, 5.4 mL). The number of procedures using the NBCA-Lipiodol mixture through microcatheter ranged from one to six sessions (mean, 4.2 sessions). As seen on venography performed after the procedure, six patients had complete obliteration of the gastric fundal varices with use of the NBCA-Lipiodol mixture (Fig. 1). One patient with a large pericardiophrenic draining vein experienced incomplete filling of the NBCA-Lipiodol mixture into the gastric varices. However, a large pericardiophrenic draining vein and multiple afferent veins were completely obliterated after use of the microcoils and the NBCA-Lipiodol mixture. Final venography did not show contrast filling into the gastric varices and draining vein (Fig. 2). No complications associated with percutaneous transportal sclerotherapy were encountered. In all of the patients, follow-up CT imaging performed after six months showed complete obliteration of the varices by the NBCA-Lipiodol mixture or by formation of thrombi. Plain chest PA X-rays did not detect any pulmonary artery embolization of the NBCA-Lipiodol mixture during follow-up.
In all patients, neither recurrent gastric varices nor variceal bleeding was observed during the follow-up period of 7 to 21 months (mean, 14.8 months). Aggravation of esophageal varices was observed in four patients and the patients were treated by endoscopic sclerotherapy. Ascites were detected in two patients and pleural effusion was detected in two patients. However, these complications were temporary.
Changes in the serum levels of laboratory parameters of the patients are presented in Table 3. The serum albumin level increased and the ammonia level decreased at six months (p < 0.05). A significant increase in the prothrombin time was observed at six months (p < 0.05). Significant changes were not seen in the bilirubin level.
The majority of gastric varices that are located at the fundus drain into the inferior pherenic vein, which later joins with the left renal vein to form the gastrorenal shunt or with the inferior vena cava just below the diaphragm to form the gastrocaval shunt (20, 24). The BRTO procedure was introduced based on obliteration for a portal-systemic venous shunt. Since its introduction by Kanagawa et al. (15), BRTO has become widely accepted as a minimally invasive, highly effective treatment for gastric varices (15-23). However, a few gastric varices cases without catheterizable main draining veins cannot be treated by BRTO. These patients can be considered for treatment with procedures such as endoscopic sclerotherapy, the use of a TIPS or percutaneous transhepatic sclerotherapy.
Endoscopic injection of NBCA is the only endoscopic treatment that has been shown as effective for gastric varices (4-6). The efficacy of endoscopic sclerotherapy for the initial hemostasis of gastric variceal bleeding has been reported as 83-100%; however, relatively high rates of rebleeding (20-25%) have also been reported (4-6). The occurrence of systemic embolization with NBCA injection has also been reported (6-9). Risk factors include a large volume injection and the existence, albeit rare, of shunts between the portal system and the pulmonary vein. However, although systemic embolization following endoscopic injection of NBCA in patients without a portosystemic shunt is a rare event, rebleeding of gastric varices remains a limitation of the use of endoscopic sclerotherapy.
A TIPS has been widely used to treat patients suffering with variceal bleeding or refractory ascites from portal hypertension (25, 26). Tripathi et al. (27) performed a comprehensive retrospective study of the use of a TIPS for the prevention of esophageal varices and gastric varices from rebleeding. The overall survival rates after the use of TIPS were significantly better for gastric varices as compared with esophageal varices, and no difference was seen between patients with gastric varices and patients with esophageal varices with regard to the rebleeding rates (20%). The incidence of new-onset encephalopathy was 17% and the cumulative incidence of shunt insufficiency was approximately 50%. Therefore, the main drawback of the use of a TIPS is its poor primary patency rate and an intractable hepatic encephalopathy due to the increased shunt volume.
Ninoi et al. (22) performed percutaneous transhepatic sclerotherapy in patients with gastric varices without a gastrorenal shunt, in patients with gastric varices with a gastrorenal shunt and gastrocaval shunts and in patients with gastric varices not treatable by BRTO. This study described coil embolization of the afferent veins and the use of 5% ethanolamine oleate with iopamidol (EOI) as the sclerosant. However, 5% EOI cannot be used in patients who retain contrast due to the complete occlusion of afferent veins. Therefore, gastric varices without a portosystemic shunt had multiple afferent veins, and complete obliteration of the afferent veins by the use of coils is very difficult to achieve. Recently, Kiyosue et al. (24) reported the use of transportal intravariceal sclerotherapy with NBCA for gastric varices. For transportal intravariceal sclerotherapy, the NBCA-Lipiodol mixture is injected via a microcatheter in the varices with flow control coils placed in the afferent and drainage vein, which can fill all of the varices and completely obliterate the varices. Although this study was performed using transhepatic sclerotherapy in a small number of patients, follow-up gastroendoscopy showed either the disappearance or a marked decrease of the varices and neither recurrent gastric varices nor variceal bleeding were observed during the follow-up period. In our study, percutaneous transportal sclerotherapy with use of the NBCA-Lipiodol mixture and microcoils immediately showed a high success rate. In addition, the presence of gastric varices was not seen for all of the patients on follow-up endoscopy. In addition, rebleeding of the gastric varices did not occur.
The augmentation of portal blood flow with the use of BRTO can improve liver function, but the use of a TIPS results in decreased portal flow, leading to deterioration of the liver function (22, 23). Kiyosue et al. (24) have reported that the results of hepatic function tests did not improvet after transportal intravariceal sclerotherapy. However, in our study, percutaneous transportal sclerotherapy was followed by an improvement in liver function. An increase in portal flow, induced by the obliteration of the large gastric varices, may contribute to the improvement in liver function seen after transportal sclerotherapy.
Many reports, including the present series, have shown a worsening of esophageal varices due to elevated portal pressure after treatment (15, 17, 20-22). However, the worsened esophageal varices, as in this study, could be successfully treated endoscopically. The presence of ascites and pleural effusion are complications caused by elevated portal pressure (20-22). In our study, ascites was detected in one patient and pleural effusion was detected in two patients. However, these complications were only temporary.
A potential risk of the use of the percutaneous transportal approach is a risk of the peritoneal hemorrhage during and after the procedure. Transjugular sclerotherapy with a TIPS approach can be considered as an alternative approach in patients with ascites and a bleeding diathesis. In addition, the technique is invasive as compared with endoscopic injection or BRTO, but it has an advantage over endoscopic injection of NBCA as flow from the varices to the shunt can be controlled with the use of coil embolization of the afferent vein and draining vein prior to injection. Therefore, this technique carries less risk of migration of NBCA into the systemic vein, which could cause a fatal pulmonary embolism, than the use of an endoscopic injection. Most gastric varices are formed by the left gastric, posterior gastric or short gastric veins (28, 29) and have multiple small branches that are connected with the main afferent veins. Therefore, multiple procedures are needed until the entire variceal complex is opacified. In our study, the number of small afferent branches obliterated with use of the NBCA-Lipiodol mixture ranged from one to six (mean, 4.2).
This study has some limitations. One limitation is that the study consited of a very small population. BRTO is the first choice of treatment for gastric varices if this procedure is possible. If BRTO is not possible, then percutaneous transportal sclerotherapy is an additional option for the treatment of gastric varices. Another limitation of the study is the lack of long-term follow-up after two years.
In conclusion, BRTO can effectively control gastric varices in patients with a portosystemic shunt. However, the use of BRTO is limited for patients without catheterizable main draining veins. Although the number of patients included in this study was small, we have demonstrated that gastric varices with can be also successfully treated with percutaneous transportal sclerotherapy and liver function can be improved. Therefore, percutaneous transportal sclerotherapy with NBCA is a good alternative treatment for patients with gastric varices without catheterizable main draining veins. However, further studies with a larger number of patients are required to confirm the findings.
Figures and Tables
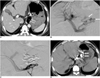 | Fig. 150-year-old man with gastric varices.
A. Contrast-enhanced CT scan shows gastric fundal varices (arrows) without portosystemic shunts (not shown). Note insertion of Sengstaken-Blakemore tube into esophagus and stomach due to active hemorrhage (open arrows).
B. Percutaneous transportal portogram shows gastric varices (arrows) draining into paraesophageal veins (not shown).
C. After 5-Fr catheter was placed into left gastric vein, NBCA-Lipiodol mixture was injected via six microcatheters. Three coils were placed in afferent veins after complete filling of NBCA-Lipiodol mixture into gastric varices.
D. Follow-up CT scan obtained six months later shows complete obliteration of varices (arrows).
|
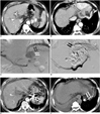 | Fig. 269-year-old man with severe gastric varices draining into large pericardiophrenic vein.
A. Contrast-enhanced CT scan shows large gastric fundal varices (arrows) without portosystemic shunts (not shown).
B. Contrast-enhanced CT scan shows draining vein of pericardiophrenic vein draining into coronary sinus (arrows).
C. Posterior gastric venogram shows large gastric varices (arrows) draining into pericardiophrenic veins (open arrows).
D. After microcatheter was placed into posterior gastric vein (afferent vein) and pericardiophrenic vein (drainage vein), 19 coils were placed (arrows). NBCA-Lipiodol mixture was injected via microcatheter after reduction of blood flow. Embolization with additional coils and injection of NBCA-Lipiodol mixture were performed in left gastric veins and short gastric veins (afferent veins).
E. CT scan obtained six months after treatment shows complete obliteration of varices (arrows).
F. Follow-up CT scan obtained six months later shows complete obstruction of draining vein due to presence of coils and NBCA-Lipiodol mixture (arrows).
|
Table 3
Clinical Results of Seven Patients

Note.-PT = prothrombin time, SD = standard deviation, *Statistically significant as compared with each value before treatment (p < 0.05).
1Changes of ascites and pleural effusion after one week.
2Changes in serum levels of laboratory measured values for patients treated with percutaneous transportal sclerotherapy after six months.
ACKNOWLEDGEMENTS
This study was supported by funds of the Chonbuk National University Hospital Research Institute of Clinical Medicine. None of the authors has identified a conflict of interest.
References
1. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992. 16:1343–1349.
2. de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001. 5:645–663.
3. Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986. 32:264–268.
4. Ramond MJ, Valla D, Mosnier JF, Degott C, Bernuau J, Rueff B, et al. Successful endoscopic obturation of gastric varices with butyl cyanoacrylate. Hepatology. 1989. 10:488–493.
5. Oho K, Iwao T, Sumino M, Toyonaga A, Tanikawa K. Ethanolamine oleate versus butyl cyanoacrylate for bleeding gastric varices: a nonrandomized study. Endoscopy. 1995. 27:349–354.
6. Ogawa K, Ishikawa S, Naritaka Y, Naritaka Y, Shimakawa T, Wagatsuma Y, et al. Clinical evaluation of endoscopic injection sclerotherapy using n-butyl-2-cyanoacrylate for gastric variceal bleeding. J Gastroenterol Hepatol. 1999. 14:245–250.
7. Snady H. The role of sclerotherapy in the treatment of esophageal varices: personal experience and a review of randomized trials. Am J Gastroenterol. 1987. 82:813–822.
8. Korula J, Chin K, Ko Y, Yamada S. Demonstration of two distinct subsets of gastric varices. Observations during a seven-year study of endoscopic sclerotherapy. Dig Dis Sci. 1991. 36:303–309.
9. Naga M, Foda A. An unusual complication of histoacryl injection. Endoscopy. 1997. 29:140.
10. See A, Florent C, Lamy P, Levy VG, Bouvry M. Cerebrovascular accidents after endoscopic obturation of esophageal varices with isobutyl-2-cyanoacrylate in 2 patients. Gastroenterol Clin Biol. 1986. 10:604–607.
11. Benedetti G, Sablich R, Lacchin T, Masiero A. Endoscopic treatment of bleeding duodenal varices by bucrylate injection. Endoscopy. 1993. 25:432–433.
12. Rossle M, Haag K, Ochs A, Sellinger M, Noldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic shunt-shunt procedure for variceal bleeding. N Engl J Med. 1994. 330:165–171.
13. LaBerge JM, Somberg KA, Lake JR, Gordon RL, Kerlan RK Jr, Ascher NL, et al. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995. 108:1143–1151.
14. Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, et al. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997. 112:889–898.
15. Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996. 11:51–58.
16. Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001. 12:327–336.
17. Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999. 211:349–356.
18. Kitamoto M, Imamura M, Kamada K, Aikata H, Kawakami Y, Kurihara Y, et al. Balloon-occluded retrograde transvenous obliteration of gastric fundal varices with hemorrhage. AJR Am J Roentgenol. 2002. 178:1167–1174.
19. Takahashi K, Yamada T, Hyodoh H, Yishikawa T, Katada R, Nagasawa K, et al. Selective balloon-occluded retrograde sclerosis of gastric varices using a coaxial microcatheter system. AJR Am J Roentgenol. 2001. 177:1091–1093.
20. Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996. 167:1317–1320.
21. Sonomura T, Sato M, Kishi K, Terada M, Shioyama Y, Kimura M, et al. Balloon-occluded retrograde transvenous obliteration for gastric varices: a feasibility study. Cardiovasc Intervent Radiol. 1998. 21:27–30.
22. Ninoi T, Nakamura K, Kaminou T, Nishida N, Sakai Y, Kitayama T, et al. TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol. 2004. 183:369–376.
23. Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003. 4:109–116.
24. Kiyosue H, Matsumoto S, Yamada Y, Hori Y, Okino Y, Okahara M, et al. Transportal intravariceal sclerotherapy with N-butyl-2-cyanoacrylate for gastric varices. J Vasc Interv Radiol. 2004. 15:505–509.
25. Cello JP, Ring EJ, Olcott EW, Koch J, Gordon R, Sandhu J, et al. Endoscopic sclerotherapy compared with percutaneous transjugular intrahepatic portosystemic shunt after initial sclerotherapy in patients with acute variceal hemorrhage. A randomized, controlled trial. Ann Intern Med. 1997. 126:858–886.
26. Rossle M, Deibert P, Haag K, Ochs A, Olschewski M, Siegerstetter V, et al. Randomised trial of trnasjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet. 1997. 349:1043–1049.
27. Tripathi D, Therapondos G, Jackson E, Redhead DN, Hayes PC. The role of the tansjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002. 51:270–274.
28. Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Correlation between endoscopic and angiographic findings in patients with esophageal and isolated gastric varices. Dig Surg. 2001. 18:176–181.
29. Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices: a study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988. 95:434–440.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download