1. Vogl TJ, Pegios W, McMahon C, Balzer J, Waitzinger J, Pirovano G, et al. Gadobenate dimeglumine - a new contrast agent for MR imaging: preliminary evaluation in healthy volunteers. AJR Am J Roentgenol. 1992. 158:887–892.
2. Lee JM, Kim IH, Kwak HS, Youk JH, Han YM, Kim CS. Detection of small hypervascular hepatocellular carcinomas in cirrhotic patients: comparison of superparamagnetic iron oxide-enhanced MR imaging with dual-phase spiral CT. Korean J Radiol. 2003. 4:1–8.
3. Tanimoto A, Wakabayashi G, Shinmoto H, Nakatsuka S, Okuda S, Kuribayashi S. Superparamagnetic iron oxide-enhanced MR imaging for focal hepatic lesions: a comparison with CT during arterioportography plus CT during hepatic arteriography. J Gastroenterol. 2005. 40:371–380.
4. Kim MJ, Kim JH, Chung JJ, Park MS, Lim JS, Oh YT. Focal hepatic lesions: detection and characterization with combination gadolinium - and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2003. 228:719–726.
5. Ward J, Guthrie JA, Scott DJ, Atchley J, Wilson D, Davies MH, et al. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology. 2000. 216:154–162.
6. Seneterre E, Taourel P, Bouvier Y, Pradel J, Van Beers B, Daures JP, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996. 200:785–792.
7. Fretz CJ, Elizondo G, Weissleder R, Hahn PF, Stark DD, Ferrucci JT Jr. Superparamagnetic iron oxide-enhanced MR imaging: pulse sequence optimization for detection of liver cancer. Radiology. 1989. 172:393–397.
8. Van Beers BE, Lacrosse M, Jamart J, Grandin C, Gigot JF, Horsmans Y, et al. Detection and segmental location of malignant hepatic tumors: comparison of ferumoxides-enhanced gradient-echo and T2-weighted spin-echo MR imaging. AJR Am J Roentgenol. 1997. 168:713–717.
9. Kim SH, Choi D, Lim JH, Lee WJ, Jang HJ, Lim HK, et al. Optimal pulse sequence for ferumoxides-enhanced MR imaging used in the detection of hepatocellular carcinoma: a comparative study using seven pulse sequences. Korean J Radiol. 2002. 3:87–97.
10. Ward J, Chen F, Guthrie JA, Wilson D, Lodge JP, Wyatt JI, et al. Hepatic lesions detection after superparamagnetic iron oxide enhancement: comparison of five T2-weighted sequences at 1.0 T by using alternative-free response receiver operating characteristic analysis. Radiology. 2000. 214:159–166.
11. Edmondson HA, Steiner PE. Primary carcinoma of liver: study of 100 cases among 48,900 necropsies. Cancer. 1954. 7:462–503.
12. Lencioni R, Pinto F, Armillotta N, Di Giulio M, Gaeta P, Di Candio G, et al. Intrahepatic metastatic nodules of hepatocellular carcinoma detected at lipiodol CT: imaging-pathologic correlation. Abdom Imaging. 1997. 22:253–258.
13. Kim MJ, Kim JH, Choi JY, Park SH, Chung JJ, Kim KW, et al. Optimal TE for SPIO-enhanced gradient-recalled echo MRI for the detection of focal hepatic lesions. AJR Am J Roentgenol. 2006. 187:W255–W266.
14. Chakraborty DP, Winter LH. Free-response methodology: alternate analysis and a new observer-performance experiment. Radiology. 1990. 74:873–881.
15. Metz CE. ROC methodology in radiologic imaging. Invest Radiol. 1986. 21:720–733.
16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.
17. Tanimoto A, Yuasa Y, Shinmoto H, Jinzaki M, Imai Y, Okuda S, et al. Superparamagnetic iron oxide-mediated hepatic signal intensity change in patients with and without cirrhosis: pulse sequence effects and Kupffer cell function. Radiology. 2002. 222:661–666.
18. Duda SH, Laniado M, Kopp AF, Gronewaller E, Aicher KP, Pavone P, et al. Superparamagnetic iron oxide detection of focal liver lesions at high-field-strength MR imaging. J Magn Reson Imaging. 1994. 4:309–314.
19. Yoshikawa T, Mitchell DG, Hirota S, Ohno Y, Oda K, Maeda T, et al. Gradient - and spin-echo T2-weighted imaging for SPIO-enhanced detection and characterization of focal liver lesions. J Magn Reson Imaging. 2006. 23:712–719.
20. Tanimoto A, Oshio K, Suematsu M, Pouliquen D, Stark DD. Relaxation effects of clustered particles. J Magn Reson Imaging. 2001. 14:72–77.
21. Tanimoto A, Kuribayashi S. Application of superparamagnetic iron oxide to imaging of hepatocellular carcinoma. Eur J Radiol. 2006. 58:200–216.
22. Carpenter KD, Macaulay SE, Schulte SJ, Obregon RG, Nelson RC, Simon HE, et al. MR of focal liver lesions: comparison of breath-hold and non-breath-hold hybrid RARE and conventional spin-echo T2-weighted pulse sequences. J Magn Reson Imaging. 1996. 6:596–602.
23. Kanematsu M, Hoshi H, Murakami T, Itoh K, Hori M, Inaba Y, et al. Fat-suppressed T2-weighted MR imaging of hepatocellular carcinoma and metastases: comparison of conventional spin-echo, fast spin-echo, and echoplanar pulse sequences. J Magn Reson Imaging. 1999. 10:25–32.
24. Alger JR, Harreld JH, Chen S, Minotorovitch J, Lu DS. Time-to-echo optimization for spin echo magnetic resonance imaging of liver metastasis using superparamagnetic iron oxide particles. J Magn Reson Imaging. 2001. 14:586–594.
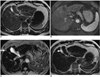
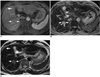
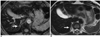
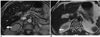






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download