Abstract
Objective
We aimed to compare the prognoses of patients with pathologically true negative (P-TN) N2 and PET/CT false negative (FN) results in stage T1 non-small cell lung cancer (NSCLC).
Materials and Methods
Our institutional review board approved this retrospective study with a waiver of informed consent. The study included 184 patients (124 men and 60 women; mean age, 59 years) with stage T1 NSCLC who underwent an integrated PET/CT and surgery. After estimating the efficacy of PET/CT for detecting N2 disease, we determined and compared disease-free survival (DFS) rates in three groups (P-TN [n = 161], PET/CT FN [n = 12], and PET/CT true positive [TP, n = 11]) using the Kaplan-Meier analysis and log-rank test.
Results
Pathologic N2 disease was observed in 23 (12%) patients. PET/CT had an N2 disease detection sensitivity of 48% (11 of 23 patients), a specificity of 95% (153 of 161), and an accuracy of 89% (164 of 184). The 3-year DFS rate in the PET/CT FN group (31%, 95% confidence interval [CI]; 13.6-48.0%) was similar to that of the TP group (16%, 95% CI; 1.7-29.5%) (p = 0.649), but both groups had significantly shorter DFS rates than the P-TN group (77%, 95% CI; 72.0-81.2%) (p < 0.001).
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is regarded as a more sensitive test than CT in the identification of malignant tissues due to alterations in tissue metabolism, which generally precede anatomic changes (1). However, PET and integrated PET/CT have a low sensitivity (approximately 50%) for mediastinal nodal staging in the T1 non-small cell lung cancer (NSCLC) stage (2, 3). This low sensitivity is likely attributed to the inability to detect early stage or microscopic lymph node metastases.
Patients with mediastinal lymph node metastases identified at mediastinoscopy have different survival rates than metastases identified at thoracotomy only. Specifically, the thoracotomy metastasis patients have a substantially better 5-year survival rate (24%) than mediastinal lymph node patients (survival rate, 9%) (4).
As mentioned above, it can be presumed that patients with a false negative (FN) FDG PET result for mediastinal nodal metastasis have early stage or microscopic mediastinal nodal metastases that cannot be identified by PET or PET/CT. However, we have no empirical evidence that these unidentified mediastinal nodal metastases automatically translate to higher survival rates (as is the case for patients with N2 disease identified at the thoracotomy but not at mediastinoscopy) compared to patients with a true positive (TP) PET result. Thus, the purpose of our study was to compare the prognoses of patients with pathologically true negative (P-TN) N2 and PET/CT FN results in stage T1 NSCLC.
Our institutional review board approved our retrospective study with a waiver of informed consent.
From September 2003 to July 2006, we enrolled 199 patients who underwent integrated PET/CT and surgical staging and were diagnosed with stage T1 NSCLC at CT (retrospective review and reanalysis of all CT data obtained during this period) or on pathologic examination. Because bronchioloalveolar carcinomas usually do not have nodal or extrathoracic metastases, we excluded patients with that type of tumor (nodule of pure ground-glass opacity). For patients with overt extra-thoracic metastases in which a conventional staging workup was not suggested, (clinical examination or enhanced thoracic CT covering down to the level of middle portion of the kidneys), a PET/CT was performed as a further pretreatment staging workup.
At our institution, patients with biopsy-proved N2 disease at mediastinoscopy are usually treated by using neoadjuvant concurrent chemo-radiation therapy (weekly taxane/platinum and 45 Gy of radiation therapy over a 5 week period) and a subsequent surgical resection. Those identified as negative for mediastinal nodal metastases at mediastinoscopy, and subsequently found to have positive mediastinal nodes after surgical resection, were administered adjuvant chemotherapy (cisplatin-based chemotherapy, three to four cycles of platinum combined with either taxane or vinorelbine). In node-negative patients at both a mediastinoscopy and thoracotomy, a curative surgical resection was performed without any adjuvant therapy.
Of these 199 patients, 10 were excluded because they did not undergo surgical treatment. In addition, another five patients were excluded because of death caused by an unrelated disease (post-operative adult respiratory distress in two, chronic empyema in the lobectomy space and its related pneumonia and sepsis in one, advanced gastric cancer in one, and acute pulmonary thromboembolism in one) during the follow-up period. The remaining patients make up the total of 184 patients included in the study (124 men and 60 women; mean age, 59 + 10 years [standard deviation]; range, 32-81 years) (Fig. 1).
Details of the imaging methods used have been described in previous reports (1-3). Briefly, peripheral blood glucose was < 150 mg/dL in all patients. Patients received an intravenous injection of 370 MBq (10 mCi) of FDG and then rested for over 45 minutes before scanning. Scans were acquired using a PET/CT unit (Discovery LS; GE Healthcare, Milwaukee, WI) consisting of a PET scanner (Advance NXi; GE Healthcare,) and an eight-slice CT scanner (LightSpeed Plus; GE Healthcare). A CT was performed from the head to the pelvic floor according to a standard protocol using the following settings: 140 kVp; 80 mA; tube rotation time, 0.5 seconds per rotation; slice pitch, 6; and section thickness, 5 mm (to match the PET section thickness). Each patient maintained normal shallow respiration during the acquisition of CT and PET scans. Immediately after an unenhanced CT, an emission PET was performed in the identical transverse field of view. The PET data sets were reconstructed iteratively using an ordered subset expectation maximization algorithm and by applying the segmented attenuation correction (two iterations, 28 subsets) to the CT data. The co-registered images were displayed using Xeleris software (GE Healthcare).
Integrated PET/CT images were evaluated jointly and prospectively by a chest radiologist (with 18 years of CT interpretation experience and 4 years of PET/CT interpretation) and a nuclear medicine physician (with 13 years of experience and 4 years of PET/CT analysis). Both were unaware of the findings of clinical and pathological evaluations. Primary tumor size was determined by measuring the largest diameter of the primary tumor on transverse lung window (window width, 1,500 HU; window level, -700) images seen at the CT component of the PET/CT images. The maximum standardized uptake values (mSUVs) of primary tumors were measured by placing an region of interest.
Nodal stations were assessed visually by allocating mediastinal lymph nodes to nine groups, according to the lymph node map definition for lung cancer staging proposed by Mountain and Dresler (5). All lymph nodes in the thorax with abnormal FDG uptake (greater than mediastinal blood pool uptake), irrespective of their size, were considered metastatic. Enlarged lymph nodes with their short-axis diameter > 10 mm were designated as benign when they were negative at the PET component of PET/CT images. Additionally, lymph nodes even with high FDG uptake, when they showing higher attenuation than mediastinal structures (great vessels) or benign calcification (central, nodular, diffuse, laminated or popcorn-like), were regarded as being benign (1-3).
During a mediastinoscopy, one of three surgeons (19, 14, and 6 years of experience, respectively) attempted to harvest entire nodes, and the American Thoracic Society (ATS) lymph node map areas of 2R, 4R, 2L, 4L, and 7 were routinely sampled. During a thoracotomy, the surgeons sampled all visible and palpable lymph nodes that were accessible in the hilum and mediastinum according to our surgical protocol. Namely, all encountered lymph nodes were removed from the ATS lymph node map areas 10R, 9, 8, 7, 4R, 3 and 2R in tumors of the right lung, and from map areas 10L, 9, 8, 7, 6, 5 and 4L of the left lung. When necessary, especially when imaging results suggested the presence of possible nodal metastasis in nodal stations of group 1 (highest mediastinal) or 2L (when tumor was located in the left lung) nodes, the nodes were also evaluated during a mediastinoscopy or thoracotomy.
A lung pathologist with 13 years of experience evaluated the nodes as numbered in the surgical field (surgeons labeled dissected lymph nodes by numbering the nodes according to the lymph node map definition for lung cancer staging) (5).
We subdivided patients into three groups according to PET/CT and pathologic findings for mediastinal lymph node metastasis, i.e., the pathologically true negative (P-TN = TN + FP at PET/CT), PET/CT FN, and PET/CT TP groups.
A follow-up evaluation was performed every three months for the first two years after operation and six months thereafter. A chest CT covering the whole thorax and upper abdomen was routinely performed at each follow-up study. If patients became symptomatic or demonstrated abnormal laboratory findings, appropriate tests (i.e., brain MR, spine MR and bone scintigraphy) were also performed. The median follow-up time following surgery in the 184 patients was 23 months (range, 10-44 months).
The statistical analysis was performed using the SPSS statistical software version 9.0 (SPSS Inc, Chicago, IL). The characteristics of the patients in the P-TN, PET/CT FN, and PET/CT TP groups were compared using the Fisher's exact test or the Chi-square test. The nonparametric data were analyzed by using the Kruskal-Wallis test. The differences in nodule size (< 2 cm versus > 2 cm in diameter) of primary cancers between N2 and N0/N1 patients were compared by using the Chi-square test.
Disease-free survival (DFS) and overall survival (OS) times were measured from the date of surgery until the first evidence of disease recurrence or last date of follow-up for patients who remained alive and were free of disease. DFS and OS were determined by using Kaplan-Meier analysis, and the differences between groups (P-TN, PET/CT FN, and PET/CT TP) groups were compared by using the log-rank test.
A univariate Cox regression analysis was used to determine whether the mSUV integer values between three and 10 were predictive of tumor recurrence. In addition, a univariate analysis was performed to evaluate the relationships between clinical or histologic prognostic factors and the probability of tumor recurrence after surgical resection. The model was adjusted for the effects of pathologic groups (P-TN, PET/CT FN, and PET/CT true positive), patient age and sex, cell type, tumor size, mSUV of the tumor, and the number of nodal stations involved. Next, a multivariate analysis was performed to identify independent prognostic determinants (Cox proportional hazards model) for both DFS and OS. Differences at the p < 0.05 level were defined as being statistically significant.
A total of 184 patients (124 men and 60 women; mean age, 59 + 10 years [standard deviation]; range, 32-81 years) were included in the study. The surgical procedures performed included a bilobectomy in three patients, a lobectomy in 179 patients, and a segmentectomy or wedge resection in two patients. Histologically, 132 patients had adenocarcinoma, 40 had squamous cell carcinoma, and 12 had other carcinomas (large cell neuroendocrine carcinomas in 5, large cell carcinoma in 4, sarcomatoid carcinoma in 2, and pleomorphic carcinoma in 1).
A mediastinoscopy was performed in 19 patients and its sensitivity for detecting N2 disease was found to be 79% (15 of 19 patients) (Fig. 1). N2 disease was observed in 23 (12%) patients. Moreover, seven of the 89 patients (8%) with smaller tumors (< 2.0 cm in diameter) and 16 of the 95 patients (17%) with tumors > 2.0 cm had N2 nodal metastases (p = 0.077). All 23 patients with N2 nodal metastases had a primary tumor with mSUVs of > 5 (mean = 9.9, (indicate unit of measure) range; 5.1-17.5 cm (indicate unit of measure)). The remaining 161 patients without nodal metastases had a primary tumor with a mean mSUV of 7.06 (range; 0.4-22.5) (p = 0.003, Mann-Whitney test).
Neoadjuvant therapy was performed in 15 patients in whom a mediastinoscopy revealed a malignant lymph node diagnosis. In these patients, a thoracotomy was followed by the neoadjuvant therapy. Next, postoperative adjuvant therapy was administered in eight patients in whom a thoracotomy pathology revealed a malignant mediastinal node diagnosis (Fig. 1).
On a per-patient basis, the PET/CT had a diagnostic efficacy for the detection of N2 disease of; sensitivity (48%, 11/23), specificity (95%, 153/161), accuracy (89%, 164/184), and positive and negative predictive values (68%, 11/19; 93%, 153/165, respectively).
A total of 39 (21%) of the 184 patients in the study experienced tumor recurrence. The recurrent sites were the lungs in 13 patients, mediastinal lymph nodes in eight, brain in six, lung and mediastinal nodes in four, bone in four, and chest wall, trachea, adrenal gland, and liver in one each. Table 2 shows the recurrence rates in terms of patient characteristics by way of a univariate analysis of the hazard ratios. Only the presence of mediastinal node metastasis and high mSUVs were found to be significantly correlated with the presence of tumor recurrence. An mSUV cutoff value of five proved to be the most discriminative criteria of tumor recurrence, based on log-rank probability values. The number of mediastinal nodal stations involved with metastasis was not found to be a predictor of tumor recurrence (four of seven patients [57%], with a single nodal station metastasis experiencing a recurrence and nine [56%] of 16 patients with multiple nodal station metastases experiencing a recurrence; yielding a hazard ratio of 1.402, p = 0.577).
The factors achieving statistical significance by multivariate analyses are summarized in Table 3. Both the presence of mediastinal node metastases and an mSUV of > 5 were found to be independent predictors of patient survival.
The three-year OS rate in the PET/CT FN group (43%, 95% confidence interval [CI]; 25.0 to 60.0%) was similar to that of the TP group (49%, 95% CI; 29.9 to 67.5%) (p = 0.863), however both groups had significantly lower OS rates than the P-TN group (90%, 95% CI; 86.5 to 93.1) (p < 0.001) (Fig. 4). Moreover, the three-year DFS rate in the PET/CT FN group (31%, 95% CI; 13.6 to 48.0%) was not statistically different from that of the TP group (16%, 95% CI; 1.7 to 29.5%) (p = 0.649); however, both groups had significantly lower DFS rates than the P-TN group (77%, 95% CI; 72.0 to 81.2%) (p < 0.001) (Fig. 5).
We found that the median OS in the PET/CT FN group (29 months) was similar to the median OS time determined for the TP group (28 months) (p = 0.863); however, both groups had significantly shorter median OS times than the P-TN group (41 months) (p < 0.001). In addition, the median DFS in the PET/CT FN group (16 months) was lower than the P-TN group (35 months) (p < 0.001); however, no significant difference was found between the FN and TP groups (12 months) (p = 0.649). Therefore, patients diagnosed with pathologic mediastinal nodal metastasis not detected by PET/CT had poorer survival estimates than patients with pathologically negative nodes. These findings suggest that a more sensitive and accurate measure should be devised to correctly triage patients with stage T1 NSCLC in terms of selecting a treatment modality and predicting prognosis.
In our patient cohort, patient age, cell type, and primary tumor size had no significant impact on OS. By both the univariate and multivariate analyses, the presence of pathologic N2 disease and a primary tumor with an mSUV > 5.0 were found to be related to the recurrence of the disease.
Some disagreement exists concerning the relationship between survival and the mSUVs of primary tumors (6-17), which is probably attributable to the various identifying methods used along with the related factors, such as, disease stages (wide range of primary tumor sizes), tumor staging accuracy, and the FDG uptake measures and analytical methods used. In our study, which addressed stage T1 lung cancers (< 30 mm in diameter) with an mSUV of > 5.0, did appear to be significantly predict a poor prognosis by using a multivariate Cox proportional hazard analysis.
It has been suggested that patients with a high SUV have poorer DFS (6-10). Previous studies have shown that different mSUV cut-off values ranging from 3.3 to 10 are useful for predicting tumor recurrence (10-13). The difference in cutoff values between our results and those of previous studies can be explained by differences in cancer stages and NSCLC histology. It can be reasonably presumed that patients with an advanced stage of lung cancer have a higher SUV and poorer prognosis than those with an early stage form of the disease. Moreover, it has been reported that the relationship between FDG uptake and tumor aggressiveness is significant in adenocarcinoma cases, but not in other histologic types of NSCLC (14-16). In contrast, Vesselle et al. (17) demonstrated that the FDG uptake values of the primary NSCLCs do not provide additional prognostic information beyond tumor size and stage. Further, they reported that mSUV is positively correlated with cancer stage and tumor size, but not directly with patient prognosis. When measuring the mSUVs of tumors < 28 mm in diameter (18, 19), the effects of limited PET reconstructed resolution and artificially lowered tumor uptake value on the mSUVs should be considered. Even after measuring partial-volume-corrected tumor mSUVs, they still found no positive relationship between mSUV and patient prognosis. In our study, an mSUV of > 5.0 will help predict tumor recurrence. However, cell types (adenocarcinoma versus non-adenocarcinoma; squamous versus non-squamous carcinomas) were not a significant prognosis determinant.
Primary tumor size is a recognized significant independent prognostic factor in patients with NSCLC. Recently, several studies have suggested that tumors < 2 cm have a better prognosis than those of > 2 cm (20-23). In our study, tumor size did not attain statistical significance by multivariate analysis. Nevertheless, although no statistical significance was not found, tumors of > 2 cm had more mediastinal nodal metastases than those < 2 cm (p = 0.077). This lack of statistical significance, despite the presence of a trend toward an effect for size > 2.0 cm, may be due to the small number of our cohort and the small number of node positive patients (12%, 23 of 184 patients).
In our study, the PET/CT diagnostic efficacy for the identification of N2 disease was based on sensitivity (48%, 11 of 23 patients), specificity (95%, 153 of 161), and accuracy (89%, 164 of 184). The low sensitivity of PET/CT for detecting N2 disease in patients with stage T1 lung cancer has already been reported (3, 4), and may be explained by the fact that we only included patients with early stages NSCLC (stage T1 cancer) and who had undergone a curative surgical resection.
Our study suffers from several limitations. First, it may include a selection bias. To aid the prognostic determination, we excluded patients who had not undergone surgical treatment and those who had died of diseases not related to lung cancer. This may have distorted the evaluation of the efficacy of PET/CT in terms of its detection of mediastinal nodal metastasis. Second, although we tried to perform a comprehensive review of the clinical and tumor histology-related factors related to prognosis, some potential factors were omitted (e.g., performance status, tumor lymphatic or vascular invasion by pathologic examination). Finally, the follow-up period was relatively short (maximum 44 months).
In conclusion, the PET/CT was found to have high a specificity and negative predictive values, but low sensitivity for detecting N2 disease in patients with a stage T1 NSCLC. In addition, patients with PET/CT FN N2 disease have survivals that are similar to those of PET/CT TP N2 disease patients, and which are both substantially shorter than those of P-TN patients. Keeping in mind our study results, we propose the following diagnostic and treatment strategy for stage T1 NSCLCs: when PET/CT suggests the presence of mediastinal nodal metastasis, mediastinoscopy should be performed to confirm the presence of N2 disease. Next, treatment plan (positive results render neoadjuvant chemoradiation therapy with or without following surgery, whereas negative results render curative resection with or without following adjuvant therapy) should be determined according to the mediastinoscopy results. When the PET/CT results suggest the absence of mediastinal nodal metastasis, direct curative resection surgery is executed with or without following adjuvant therapy according to the pathologic results of the curative surgery.
Figures and Tables
Fig. 1
Flowchart illustrating study design and number of patients enrolled in this study from each group. P-TN = pathologically true negative, TN = true negative, FP = false positive, FN = false negative, TP = true positive, *Neither FDG uptake amount of primary tumor or PET/CT mediastinal nodal FDG uptake result was determinant for performing mediastinoscopy.

Fig. 2
False negative PET/CT interpretation for mediastinal nodal staging in 42-year-old woman with stage T1 adenocarcinoma of lung showing recurrent disease on follow-up examination.
A, B. Transverse (A) and coronal (B) images of initial PET/CT show 20-mm-sized nodule (arrows) in right lower lobe (maximum SUV = 9.5). There was no identifiable mediastinal uptake, but thoracotomy disclosed malignant cells in right lower paratracheal (nodal station 4R) and subcarinal (station 7) nodes.
C, D. 9-month follow-up transverse CT (C) and coronal PET (D) scans demonstrate 20-mm-sized right anterior diaphragmatic node (arrows) with high amount FDG uptake (maximum SUV = 7.0), which is suggestive of recurrent disease.
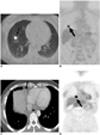
Fig. 3
True positive PET/CT interpretation for mediastinal nodal staging in 46-year-old man with stage T1 adenocarcinoma of lung showing metastatic disease on follow-up examination.
A, B. Transverse images of initial PET/CT show 29-mm-sized nodule (arrow in A) with high amount of FDG uptake (maximum SUV = 10.4) in right lower lobe and right lower paratracheal lymph node (nodal station 4R, arrow in B) of high FDG uptake. Nodes contained malignant cells upon examination of mediastinoscopic biopsy.
C, D. Initial (C) and follow-up (D) enhanced sagittal T1-weighted MR images over seven month follow-up period show newly developed metastatic nodule (arrow in D) in cerebellar vermis.
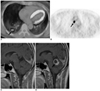
Fig. 4
Diagram illustrating comparison of overall survival of 184 patients belonging to pathologic true negative (TN), PET/CT false negative (FN), and true positive (TP) groups. Survival is significantly better in true negative group than false negative or true positive group.

Fig. 5
Diagram illustrating comparison of disease-free survival of 184 patients belonging to pathologic true negative (TN), PET/CT false negative (FN), and true positive (TP) groups. Disease-free survival is significantly greater in true negative group than false negative or true positive group.

Table 1
Characteristics of Patients in Pathologic True Negative, False Negative, and True Positive Groups Based on Clinicopathologic Factors

References
1. Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005. 236:1011–1019.
2. Kim BT, Lee KS, Shim SS, Choi JY, Kwon OJ, Kim H, et al. Stage T1 non-small cell lung cancer: preoperative mediastinal nodal staging with integrated FDG PET/CT - a prospective study. Radiology. 2006. 241:501–509.
3. Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, et al. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country: diagnostic efficacy in 674 patients. Cancer. 2007. 109:1068–1077.
4. Pearson FG, DeLarue NC, Ilves R, Todd TR, Cooper JD. Significance of positive superior mediastinal nodes identified at mediastinoscopy in patients with resectable cancer of the lung. J Thorac Cardiovasc Surg. 1982. 83:1–11.
5. Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997. 111:1718–1723.
6. Ahuja V, Coleman RE, Herndon J, Patz EF Jr. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer. 1998. 83:918–924.
7. Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005. 23:1136–1143.
8. Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004. 22:3255–3260.
9. Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002. 43:39–45.
10. Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005. 130:151–159.
11. Ohtsuka T, Nomori H, Watanabe K, Kaji M, Naruke T, Suemasu K, et al. Prognostic significance of [(18)F]fluorodeoxyglucose uptake on positron emission tomography in patients with pathologic stage I lung adenocarcinoma. Cancer. 2006. 107:2468–2473.
12. van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007. 43:1392–1398.
13. Vansteenkiste JF, Stroobants SG, Dupont PJ, De Leyn PR, Verbeken EK, Deneffe GJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999. 17:3201–3206.
14. Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Kobayashi T, et al. Fluorine 18-tagged fluorodeoxyglucose positron emission tomographic scanning to predict lymph node metastasis, invasiveness, or both, in clinical T1 N0 M0 lung adenocarcinoma. J Thorac Cardiovasc Surg. 2004. 128:396–401.
15. Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004. 45:19–27.
16. Sagawa M, Higashi K, Sugita M, Ueda Y, Maeda S, Toga H, et al. Fluorodeoxyglucose uptake correlates with the growth pattern of small peripheral pulmonary adenocarcinoma. Surg Today. 2006. 36:230–234.
17. Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, Hawes SE, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res. 2007. 13:3255–3263.
18. Vesselle H, Turcotte E, Wiens L, Schmidt R, Takasugi JE, Lalani T, et al. Relationship between non-small cell lung cancer fluorodeoxyglucose uptake at positron emission tomography and surgical stage with relevance to patient prognosis. Clin Cancer Res. 2004. 10:4709–4716.
19. Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with [F-18]fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res. 2000. 6:3837–3844.
20. Flieder DB, Port JL, Korst RJ, Christos PJ, Levin MA, Becker DE, et al. Tumor size is a determinant of stage distribution in t1 non-small cell lung cancer. Chest. 2005. 128:2304–2308.
21. Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest. 2003. 124:1828–1833.
22. Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg. 1994. 107:1087–1093.
23. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. 2005. 129:87–93.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download