Abstract
Aortic valvular stenosis (AS) is the most common valve disease which results in the need for a valve replacement. Although a Doppler echocardiography is the current reference imaging method, the multidetector computerized tomograpghy (MDCT) and magnetic resonance imaging (MRI) have recently emerged as a promising method for noninvasive valve imaging. In this study, we briefly describe the usefulness and comparative merits of the MDCT and MRI for the evaluation of AS in terms of valvular morphology (as the causes of AS), quantification of aortic valve area, pressure gradient of flow (for assessment severity of AS), and the evaluation of the ascending aorta and cardiac function (as the secondary effects of AS). The familiarity with the MDCT and MRI features of AS is considered to be helpful for the accurate diagnosis and proper management of patients with a poor acoustic window.
Aortic valvular stenosis (AS) is defined as a condition in which the opening of the aortic valve in the systolic phase is restricted. AS is the most common valve disease which results in valve replacement (1). This condition can be caused by a variety of disorders affecting the cusps or annuli. In infants, children, and adolescents, the major causes of AS are congenital malformations of the cusps such as bicuspid aortic valves or annuli, as well as rheumatic disease. In patients over 60 years of age, the major causes of AS include the calcification of congenitally bicuspids or normal tricuspid valves, in addition to the senile degeneration of the valve cusps or annuli (2). Once initiated, the progressive leaflet calcification and fibrosis eventually results in reduced leaflet motion accompanied by obstruction of the left ventricular outflow. Furthermore, AS is typically initially presented as an asymptomatic murmur in patients. However, the classical symptoms develop in the triad of angina, syncope, and heart failure, with only one quarter of patients surviving up to three years without valve replacement. Therefore, the determination for the accurate timing of surgery is important in AS (3). In this aspect, an echocardiography is known to be an essential method for assessing the physiological significance of AS, because it is widely available with no invasiveness and low cost. However, an echocadiography may be difficult to perform in patients with thick chest walls or chest deformities, as well as in elderly patients (4). Another diasadvantage of the echocardiography is the operater dependence. Recently, emerging tools in noninvasive cardiac imaging such as electrocardiographic (ECG) - gated multidetector row computed tomography (MDCT) and magnetic resonance imaging (MRI) is practically applied for the evaluation of valvular disease. These modalities are reported as being more reliable in the evaluation of the valvular morphology and function, as well as providing additional information such as extracardiac findings (5, 6). In this study, we illustrate the 1) valvular morphology (as the causes of AS), 2) quantification of aortic valve area (AVA) and pressure gradient of flow (to assess the severity of AS), and 3) the evaluation of the ascending aorta and cardiac function (as the secondary effects of AS) in patients with AS, by using the ECG-gated MDCT and MRI.
Because the MDCT has a higher spatial resolution than does MR imaging, the anatomic details of the valve leaflets, chordae tendinae, and papillary muscles can be properly visualized with an MDCT. However, unlike the aortic valve assessment on an echocardiography and an MRI, which derive an index of functional aortic valve area by pressure or velocity measurements, the assessment of aortic stenosis on MDCT is purely anatomic and is performed through the direct anatomic planimetry of the valve in midsystolic phase when the valve cusps are open and relatively quiescent. Lawler et al. (7) reported that the most feasible method for cardiac valve evaluation is to upload the entire 4D data set (0-100% reconstruction at 10% intervals) and use a thin-slab maximum intensity projections (MIP) or a volume rendering to create reformatted images in any plane desired.
For the aortic valve assessment with MR imaging, three principal techniques were performed including black blood imaging, steady-state free precision (SSFP) cine imaging, and phase-contrast imaging.
Black blood MR imaging remains the first step in assessing the cardiac chamber and valve morphologic features, such as the thickening of the valve leaflets (8). Cardiac chamber function and valve motion is assessed with SSFP cine MR imaging. Cardiac motion is displayed in a cine loop of 20-30 frames covering the entire R-R interval. For the phase-contrast MR imaging method, the technologist must set the flow-sensitizing gradients at a level greater than or equal to the expected peak velocity (threshold value, encoding velocity). If the blood velocities exceed the prescribed encoding velocity, aliasing artifact occurs, which substantially complicates the analysis of the phase-contrast data set (9).
The morphology of the aortic valve, including aortic valve leaflets, free edges, and annuli, can be assessed in parallel and perpendicular planes at the mid-systolic phase (i.e., open valve) and at the mid-diastolic phase (i.e., closed valve) using multiplanar reformation and double-oblique reformations (Fig. 1) on an ECG-gated MDCT. The aortic valve was normally found to be tricuspid (composed of symmetric three leaflets) (Fig. 2). However, congenitally malformed valves such as bicuspid valves (Figs. 3, 4), unicuspid valves (Fig. 5), and quadricuspid valves (Fig. 6), are more predisposed to develop calcification, stenosis, and regurgitation. Because the abnormal architecture induces turbulent flow, it traumatizes the leaflets and leads to fibrosis, increased rigidity, and calcification of the leaflets, which ultimately results in the narrowing of the aortic orifice. An ECG-gated MDCT has the ability to accurately depict these morphologic abnormalities of the aortic valve (10-12).
The presence and extent of valvular calcification in patients with AS have been identified as an important predictor of clinical outcome (13). Moreover, high aortic valve calcification scores indicate the possibility of severe aortic stenosis and should prompt a further functional evaluation (14). Consistently, past literature identified a correlation between the degree of valvular calcification and the severity of aortic stenosis (15). It is well known that the MDCT is superior to other modalities for the detection and quantification of valvular calcification. In addition, it has been validated by studying patients prior to surgery and comparing the results with examinations of the pathological specimen (15). However, a bright-blood MRI is not a reliable method for detecting the calcification of the aortic valve, because the extent of the signal void depends on the pulse sequence used, its specific parameters, and the placement of the cine sections. In addition, signal voids on an MRI caused by valvular calcification may be difficult to distinguish from the flow jets through the stenotic valves (16). Moreover, the extent of valve calcification has also been shown to be a significant predictor of outcome in AS (17, 18).
The aortic valve area and transvalvular pressure gradient are major variables used in the assessment of AS severity. Effective AVA is frequently measured to quantify the degree of aortic stenosis using an echocardiography. The MDCT allows a three-dimensional acquisition of the entire heart throughout the cardiac cycle and multiple plane reconstructions, which can be sliced in any plane as desired. It is thus possible to obtain a perfectly oriented parasternal short-axis view of the AVA. Several studies have reported a good correlation and reasonable agreement between the AVA calculated by an MDCT and an echocardiography (19, 20) (Table 1). Feuchtner et al. (19) suggested that the optimal reconstruction window for the measurement of AVA is positioned within mid-late systolic phase, which corresponds with the ejection phase in accordance with the T-wave on the ECG signal.
The MRI planes chosen for the planimetry of the AVA were orthogonal to the stenotic jet, as deduced from the area of signal loss due to the turbulent flow at the valve orifice level. The AVA measured by MR has also demonstrated a reproducible and observer-independent method which correlates well with the echocardiography (21, 22) (Table 2). Pouler et al. (6) demonstrated that the MDCT planimetric measurements of AVA are highly reproducible and correlate strongly with the MR and transechophageal echocardiography (TEE) planimetric measurements of AVA as well as with the transthoracic echocardiography (TTE) measurements of AVA obtained by using the continuity equation. Therefore, the ECG-gated MDCT and MRI provide an accurate, noninvasive imaging technique for quantification of AVA through the valve plane, which can be graded (Fig. 7).
The velocity-encoded cine (VENC) MRI used for the measurement of blood flow velocity and volume flow provides an accurate estimate of the transvalvular pressure gradients in many clinical situations. The peak systolic velocity depends on the angle between the flow jet and the imaging plane. Therefore, if the flow is not perpendicular to the aortic valve plane, an underestimation of the peak systolic velocity could occur. In an echocardiography with a Doppler image, poor echocardiographic windows may compromise the recording quality and unusual anatomic configurations, such as the ectatic aortas. In addition, the horizontal heart positions may preclude the exact parallel orientation of the Doppler beam with the high-velocity aortic jets. In contrast, the VENC MRI is a reliable and reproducible tool to evaluate peak systolic velocity of the stenotic aortic valves (23), because it provides the exact imaging plane parallel to the plane of the aortic valve (Fig. 8). The peak systolic velocity is used to calculate the peak pressure gradient. The pressure gradient determined using the VENC MR, correlated well with the invasive catheterization, and echocardiography (24, 25) (Table 3).
Poststenotic dilatation of the ascending aorta is a common finding in patients with severe AS. The TTE is limited to the diagnosis of aneurysms located at the ascending aorta and the quantification of aneurysm size because it could not consistently visualize the mid or distal ascending aorta. Therefore, the ECG-gated MDCT and MRI generally allows for more accurate and reliable quantification of the ascending aorta, often with more important clinical parameters than the echocardiography (Fig. 9).
A left ventricular hypertrophy is another frequent finding in patients afflicted with severe AS, which is a key adaptive mechanism to the pressure load imposed by AS. The accurate evaluation of the left ventricular systolic function and mass is important in the management of AS, because it is closely related to cardiac morbidity and mortality. The current standard of reference for left ventricular function is analysis by MRI (Fig. 10). The performance of the MRI is significantly superior to echocardiography in the interstudy reproducibility coefficient of variability used to measure cardiac function (26). In addition, the MRI does not rely on the geometric assumptions for the left ventricular function parameters as well as no ionizing radiation. In recent years, because of the extensive technological improvements in cardiac functional analysis, the MDCT has been technically possible. The data from the pooled analysis show that there is a small but systematic overestimation of the ventricular volumes by MDCT. One could contemplate that this effect is related to the lower level of contrast between blood and myocardium seen in an MDCT, or its lower number of acquired phases (27). However, the diagnostic accuracy increased with the introduction of more detector rows in the MDCT. The systolic functional analysis with the ECG-gated MDCT is also more accurate than the two-dimensional echocardiography or the ECG-gated SPECT (single photon emission computed tomography) (28).
In AS, the ECG-gated MDCT and MRI may provide the important information pertaining to valve morphology and the severity of stenosis, as well as additional findings on the ascending aorta. Even so, the role of MDCT in AS has some limitations at the present time, because it does not yield additional hemodynamic information such as transvalvular pressure gradients or the presence of regurgitation. In addition, it frequently produces motion artifact in patients with higher heart rates (29). We should also consider radiation hazard associated with this method. The MRI also has limitations in terms of its high cost, relatively long scan time, limited availability, and the poor detection of aortic valve calcification. However, in patients with inadequate and inconclusive echocardiogaphies, the MDCT and MRI may serve as an alternative for the assessment of AS (29). Another potential role of the MDCT in the assessment of AS is the pre-operative assessment of coronary arteries as an alternative to invasive coronary angiographies in patients with a low likelihood of coronary artery disease (30). Therefore, familiarity with the MDCT and MRI features of AS will be helpful for the accurate diagnosis and proper management.
Figures and Tables
Fig. 1
Plane selection for evaluation of aortic valve morphology in patient with normal aortic valve on ECG-gated multidetector CT. Note normal three cups and aortic valve in diastolic phase (A, C) and opening in systolic phase (B, D) of double-oblique reconstruction images.
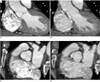
Fig. 2
54-year-old man with severe aortic stenosis. Double-oblique reconstruction image of ECG-gated multidetector CT shows calcified tricuspid valve.
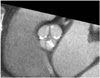
Fig. 3
Incidentally detected non-calcified bicuspid valve that on ECG-gated multidetector CT in systolic phase (A) and diastolic phase (B). Note typical "fishmouth" (arrows) appearance of bicuspid valve in systolic phase.
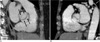
Fig. 4
Thickened bicuspid valve with severe aortic stenosis. Thickened bicuspid valve with severe aortic stenosis from ECG-gated multidetector CT in systolic phase (A) and diastolic phase (B) is well correlated with surgical findings (C).
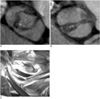
Fig. 5
Thickened unicommisural unicuspid valve with severe aortic stenosis. Identified thickened unicuspid valve from ECG-gated multidetector CT in systolic phase (A) and diastolic phase (B) is well correlated with surgical findings (C). Note raphe (thin arrows) and calcification (thick arrow) of unicuspid aortic valve.
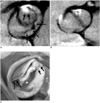
Fig. 6
Multidetector CT scan of aortic valve during diastolic phase (A) and systolic phase (B) shows three equal-sized leaflets and one smaller valve leaflets. Note incomplete coaptation of leaflets centrally (*), resulting in aortic insufficiency.
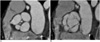
Fig. 7
Measurement of aortic valve area in patients with severe aortic stenosis. Cross-sectional view of severely stenotic tricuspid valve is used for measurement of aortic valve area in systolic phase of ECG-gated multidetector CT image (A) and MRI (B). White line denotes aortic valve area.
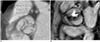
Fig. 8
65-year-old man with severe aortic stenosis and bicuspid aortic valve. Magnitude (A) and phase (B) images for flow measurements of stenotic bicuspid aortic valve using velocity encoded MRI. Line denotes aortic valve area with result of 0.85 cm2. Peak systolic velocity was measured at 547.68 cm/sec, and corresponds to peak pressure gradient of 119 mmHg.
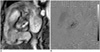
Fig. 9
67-year-old man with severe aortic stenosis. Image of ECG-gated multidetector CT (A) demonstrates post-stenotic dilatation of ascending aorta due to severe aortic stenosis. ECG-gated multidetector CT and MRI can provide accurate sizing of ascending aorta (B).
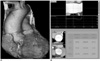
Fig. 10
64-year-old woman with severe aortic stenosis and bicuspid aortic valve. Cine MRI, (A) using steady-state free precession sequence, shows thickened aortic valve (thick small arrows) and left ventricular hypertrophy (small arrows). Flow jet (arrows) is also well visualized (B).
References
1. Selzer A. Changing aspects of the natural history of valvular aortic stenosis. N Engl J Med. 1987. 317:91–98.
2. Dare AJ, Veinot JP, Edwards WD, Tazelaar HD, Schaff HV. New observations on the etiology of aortic valve disease: a surgical pathologic study of 236 cases from 1990. Hum Pathol. 1993. 24:1330–1338.
3. Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006. 114:E84–E231.
4. Ryan EW, Bolger AF. Transesophageal echocardiography (TEE) in the evaluation of infective endocarditis. Cardiol Clin. 2000. 18:773–787.
5. Bamgartner H. Is there a role for multislice computed tomography in aortic stenosis? Eur Heart J. 2006. 27:2923–2924.
6. Pouleur AC, le Polain de Waroux JB, Pasquet A, Vanoverschelde JL, Gerber BL. Aortic valve area assessment: multidetector CT compared with cine MR imaging and transthoracic and transesophageal echocardiography. Radiology. 2007. 244:745–754.
7. Lawler LP, Ney D, Pannu HK, Fishman EK. Four-dimensional imaging of the heart based on near-isotropic MDCT data sets. AJR Am J Roentgenol. 2005. 184:774–776.
8. Arai AE, Epstein FH, Bove KE, Wolff SD. Visualization of aortic valve leaflets using black blood MRI. J Magn Reson Imaging. 1999. 10:771–777.
9. Lotz J, Meier C, Leppert A, Galanski M. Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics. 2002. 22:651–671.
10. Jacobs JE, Srichai M, Kim D, Hecht E, Kronzon I. Quadricuspid aortic valve: imaging findings on multidetector helical CT with echocardiographic correlation. J Comput Assist Tomogr. 2006. 30:569–571.
11. Alkadhi H, Wildermuth S, Plass A, Bettex D, Baumert B, Leschka S, et al. Aortic stenosis: comparative evaluation of 16-detector row CT and echocardiography. Radiology. 2006. 240:47–55.
12. Willmann JK, Weishaupt D, Lachat M, Kobza R, Roos JE, Seifert B, et al. Electrocardiographically gated multi-detector row CT for assessment of valvular morphology and calcification in aortic stenosis. Radiology. 2002. 225:120–128.
13. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000. 343:611–617.
14. Koos R, Mahnken AH, Sinha AM, Wildberger JE, Hoffmann R, Kuhl HP. Aortic valve calcification as a marker for aortic stenosis severity: assessment on 16-MDCT. AJR Am J Roentgenol. 2004. 183:1813–1818.
15. Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, et al. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004. 110:356–362.
16. Glockner JF, Johnston DL, McGee KP. Evaluation of cardiac valvular disease with MR imaging: qualitative and quantitative techniques. Radiographics. 2003. 23:E9.
17. Koos R, Kuhl HP, Muhlenbruch G, Wildberger JE, Gunther RW, Mahnken AH. Prevalence and clinical importance of aortic valve calcification detected incidentally on CT scans: comparison with echocardiography. Radiology. 2006. 241:76–82.
18. Liu F, Coursey CA, Grahame-Clarke C, Sciacca RR, Rozenshtein A, Homma S, et al. Aortic valve calcification as an incidental finding at CT of the elderly: severity and location as predictors of aortic stenosis. AJR Am J Roentgenol. 2006. 186:342–349.
19. Feuchtner GM, Dichtl W, Friedrich GJ, Frick M, Alber H, Schachner T, et al. Multislice computed tomography for detection of patients with aortic valve stenosis and quantification of severity. J Am Coll Cardiol. 2006. 47:1410–1417.
20. Feuchtner GM, Muller S, Bonatti J, Schachner T, Velik-Salchner C, Pachinger O, et al. Sixty-four slice CT evaluation of aortic stenosis using planimetry of the aortic valve area. AJR Am J Roentgenol. 2007. 189:197–203.
21. Schlosser T, Malyar N, Jochims M, Breuckmann F, Hunold P, Bruder O, et al. Quantification of aortic valve stenosis in MRI-comparison of steady-state free precession and fast low-angle shot sequences. Eur Radiol. 2007. 17:1284–1290.
22. John AS, Dill T, Brandt RR, Rau M, Ricken W, Bachmann G, et al. Magnetic resonance to assess the aortic valve area in aortic stenosis: how does it compare to current diagnostic standards? J Am Coll Cardiol. 2003. 42:519–526.
23. Caruthers SD, Lin SJ, Brown P, Watkins MP, Williams TA, Lehr KA, et al. Practical value of cardiac magnetic resonance for clinical quantification of aortic valve stenosis: comparison with echocardiography. Circulation. 2003. 108:2236–2243.
24. Eichenberger AC, Jenni R, von Schulthess GK. Aortic valve pressure gradients in patients with aortic valve stenosis: quantification with velocity-encoded cine MR imaging. AJR Am J Roentgenol. 1993. 160:971–977.
25. Sondergaard L, Stahlberg F, Thomsen C. Magnetic resonance imaging of valvular heart disease. J Magn Reson Imaging. 1999. 10:627–638.
26. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002. 90:29–34.
27. van der Vleuten PA, Willems TP, Götte MJ, Tio RA, Greuter MJ, Zijlstra F, et al. Quantification of global left ventricular function: comparison of multidetector computed tomography and magnetic resonance imaging. A meta-analysis and review of the current literature. Acta Radiol. 2006. 47:1049–1057.
28. Yamamuro M, Tadamura E, Kubo S, Toyoda H, Nishina T, Ohba M, et al. Cardiac functional analysis by multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT and MR imaging. Radiology. 2005. 234:381–390.
29. Bamgartner H. Is there a role for multislice computed tomography in aortic stenosis? Eur Heart J. 2006. 27:2923–2924.
30. Gilard M, Cornily JC, Pennec PY, Joret C, Le Gal G, Mansourati J, et al. Accuracy of multislice computed tomography in the preoperative assessment of coronary disease in patients with aortic valve stenosis. J Am Coll Cardiol. 2006. 47:2020–2024.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download