1. Armpilia CI, Fife IA, Croasdale PL. Radiation dose quantities and risk in neonates in a special care baby unit. Br J Radiol. 2002. 75:590–595.
2. Richardson RB. Past and revised risk estimates for cancer induction by irradiation and their influence on dose limits. Br J Radiol. 1990. 63:235–245.
3. Institude of Physics and Engineering in Medicine. Report No77. Recommended standards for the routine performance testing of diagnostic X-ray imaging systems. 1997. York: IPEM.
4. Chapple CL, Faulkner K, Hunter EW. Energy imparted to neonates during X-ray examinations in a special care baby unit. Br J Radiol. 1994. 67:366–370.
5. Wall BF, Harrison RM, Spiers FW. Report No. 53. Patient dosimetry techniques in diagnostic radiology. Institute of Physical Science in Medicine. 1988. York, UK: IPEM.
6. Tapiovaara M, Lakkisto M, Servomaa A. Report STUK-A139. PCXMC- A PC-based Monte Carlo Program for Calculating Patient Doses in Medical X-ray Examinations. 1997. Helsinki: Radiation and Nuclear Safety Authority (STUK).
7. International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 1991. 21:1–201.
8. European commission. Report EUR 16261 EN. European guidelines on quality criteria for diagnostic radiographic images in paediatrics. 1996. Brussels: EC.
9. Hart D, Wall BF, Schrimpton PC, Bungay DR, Dance DR. Reference doses and patient size in paediatric radiology. NRPB-R318. 2000. Chilton: HMSO.
10. Duggan L, Warren-Forward H, Smith T, Kron T. Investigation of dose reduction in neonatal radiography using specially designed phantoms and LiF: Mg, Cu, P TLDs. Br J Radiol. 2003. 76:232–237.
11. Brindhaban A, Al-Khalifah K. Radiation dose to premature infants in neonatal intensive care units in Kuwait. Radiat Prot Dosimetry. 2004. 111:275–281.
12. Jones NF, Palarm TW, Negus IS. Neonatal chest and abdominal radiation dosimetry: a comparison of two radiographic techniques. Br J Radiol. 2001. 74:920–925.
13. Robinson A, Dellagrammaticas HD. Radiation doses to neonates requiring intensive care. Br J Radiol. 1983. 56:397–400.
14. Schneider K, Kohn MM, Ernst G. The derivation of reference dose values to chest X-rays in radiography. Radiat Prot Dosim. 1998. 80:199–202.
15. Lowe A, Finch A, Boniface D, Chaudhuri R, Shekhdar J. Diagnotic image quality of mobile neonatal chest X-rays and the radiation exposure incurred. Br J Radiol. 1999. 72:55–61.
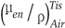 is the mass energy absorption coefficient ratio of tissue to air. The mass energy absorption coefficient ratio is equal to 1.05 for the kVp range used in this study (5).
is the mass energy absorption coefficient ratio of tissue to air. The mass energy absorption coefficient ratio is equal to 1.05 for the kVp range used in this study (5).



 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download