Abstract
We report a case of a 45-year-old man who underwent endovascular treatment in the acute setting of a subarachnoid hemorrhage due to rupture of a wide-necked basilar trunk aneurysm. The patient was treated with stent implantation without coiling. A control angiographic scan obtained immediately after the procedure revealed significantly decreased intraaneurysmal flow. Follow-up angiography performed after one month demonstrated total aneurysm occlusion.
Endovascular treatment of ruptured intracranial aneurysms has gained wide acceptance and has evolved significantly since its introduction into clinical use in the early 1990s (1).
With the development of endovascular techniques and devices, the indications for this type of treatment have also expanded. Initially, endovascular embolization was reserved for patients in poor neurological condition or for patients with aneurysms in the posterior circulation.
Currently, even fusiform or wide-necked aneurysms are no longer considered contraindicated for endovascular treatment, although wide-necked aneurysms are a great challenge for interventional radiologist. Endovascular treatment of wide-necked aneurysms carries a risk of coil protrusion into the parent vessel, incomplete occlusion or long-term angiographic recurrence (2). The application of a self-expandable stent simplifies effective coil packing into the aneurysmal sack via the stent mesh, and thus prevents prolapse.
We report a case of a 45-year-old patient with a ruptured, saccular, wide-necked aneurysm on the basilar trunk where aneurysm thrombosis occurred after stent implantation without additional coil packing.
A 45-year-old man was admitted to the hospital with a suspicion of subarachnoid hemorrhage (SAH). The patient presented with psychomotor slowdown, limited verbal contact and disorientation. The patient was in Hunt and Hess grade III. A non-contrast computerized tomography (CT) scan of the patient confirmed the presence of massive SAH (Fisher grade IV) with intraventricular hemorrhage (Fig. 1A). Shortly after admission, the patient experienced sudden cardiac arrest and the patient was resuscitated. The patient was connected to a respirator and his state was stabilized. External ventricular drainage was performed and the clinical condition improved. After removal of ventricular drainage, digital subtraction angiography (DSA) was performed. DSA demonstrated the presence of a wide-neck, lobular, saccular aneurysm on the basilar trunk between the superior cerebellar artery and the anterior inferior cerebellar artery, with a diameter of approximately 6 mm and neck length of 4 mm (Fig. 1B). DSA also revealed aplasia of the P1 segment on the left side. A decision to perform endovascular treatment was made. Considering the length of the neck and the risk associated with protrusion of the coils, a decision to perform stent implantation was made. The patient was preloaded with 150 mg acetylsalicylic acid (ASA, aspirin). In our institution, patients in acute setting of SAH are preloaded only with ASA. In our opinion, its use carries a lower risk of hemorrhagic complications during the procedure than the use of dual antiplatelet therapy with ASA and clopidogrel. The patient provided informed consent and the patient was then sedated. The procedure was performed under general anaesthesia. The patient was administered 5,000 units of heparin intravenously during the procedure to maintain an activated clotting time of 250-350 after the femoral sheath was introduced. A 6-Fr guiding catheter Casasco (Balt, Montmorency, France) was introduced into the left vertebral artery. Through the guiding catheter, a Vasco 21+ microcatheter (Balt, Montmorency, France) was navigated on a 0.014 guidewire (Balt, Montmorency, France) to the parent vessel distally to the aneurysmal neck. The delivery system of the Leo stent (Balt, Montmorency, France) was then introduced inside the Vasco microcatheter. A Leo stent is a nitinol, closed-cell, self-expandable stent dedicated to intracranial circulation. Two platinum threads along the entire length of the stent enable visualization of the stent (both the length and diameter). The stent is resheathable when up to 90% is deployed.
The 2.5×12 mm stent was successfully deployed in the basilar artery and its proximal end was placed just below the origin of the right posterior cerebral artery (Fig. 1C). Control angiography performed immediately after stent placement demonstrated significantly decreased intraaneurysmal flow due to the beginning of intraaneurysmal thrombosis (Fig. 1D) and no coil packing was performed and no second antiplatelet agent was administered. After the procedure, the patient condition was evaluated as poor with a decreased level of consciousness. A CT scan performed 13 days after stent insertion identified the presence of a hydrocephalus and a ventriculo-peritoneal valve was implanted. The neurological condition of the patient improved significantly and the patient was discharged with a Glasgow Outcome Scale score of 3. Antiplatelet therapy was continued with ASA for life. The decision not to administer a second antiaggregative agent after the procedure was made because the aneurysm dome was unsecured with coils. Additionally, the stent used was short (12 mm) and was not placed through the basilar bifurcation. During a 30-minute observation, blood flow within the stent and the basilar artery was normal without any signs of vasospasm.
A follow-up examination performed after one month confirmed the patency of the stent and complete occlusion of the aneurysm was seen (Fig. 1E). No additional aneurysm embolization was attempted. The patient was in good general condition and was discharged with a modified Rankin scale of 3 points.
The application of a stent into the parent artery has three main advantages. The first advantage is that application of a stent into the parent artery enables dense aneurysm packing with coils without compromising the vessels lumen (3, 4). Second, application of a stent into the parent artery alters the intra-aneurysmal flow pattern that facilitates thrombosis and reduction of coil compaction in the region of inflow zone and prevents subsequent re-growth of aneurysm (3-5). Finally, the stent mesh may provide a framework for endothelial growth, enabling remodeling of the aneurysm neck and causing its permanent separation from the parent vessel lumen (3, 4).
In the present case, intraaneurysmal flow decreased immediately after stent insertion. A follow-up angiographic scan demonstrated total aneurysm occlusion. Although planned coils embolization was not attempted, the patient was secured against rebleeding. Though patients with wide-necked aneurysms have another option of endovascular treatment (the use of the balloon-assisted technique), it is not recommended for aneurysms on the basilar artery (6). Total occlusion of the aneurysmal sack is difficult to achieve without unexpected occlusion of the parent artery (6).
Cases of thrombosis within an aneurysm sack induced by stent placement have been previously described (7). Lopes et al. (8) described cadaveric histological evaluation of a carotid artery aneurysm treated with a Neuroform stent four months after stenting. Complete endothelialization of the stent and filling of aneurysmal sack with fibrocellular tissue was demonstrated. However, there was a lack of any fibrocellular tissue in the central part of the neck with material resembling a thrombus.
A similar aneurysm occlusion obtained by double stent implantation was also described (9). The experience of Benndorf and colleagues (9) showed that reduction of stent porosity, obtained by stent in stent placement, might significantly dampen the intraaneurysmal blood flow and thus accelerate thrombosis within the aneurysmal sack. Yu and Zhao (10) also documented similar results in an in vitro study. These investigators found that flow movement inside the aneurysmal sack could be dwarfed to less than 5% of the bulk mean velocity. Moreover, regions of high wall shear stresses at the distal neck could also be dwarfed by almost 90% (10).
Based on our experience, placement of only one stent may be sometimes sufficient. However, this phenomenon is unpredictable. Immediate aneurysmal obliteration can be also observed after single stent placement for treatment of pseudoaneurysms and dissecting aneurysms (9).
Although a stent placement seems as a great option for wide-necked aneurysms, it is not free from risk of complications. Thrombogenicity of metal surfaces poses a risk of thrombosis within a stent (4). Therefore, the prevention of this event includes aggressive antiaggregative therapy with two antiplatelet drugs, usually with ASA (aspirin) and clopidogrel (3). However, in patients in the acute setting of SAH, aggressive the use of an antithrombotic regimen is controversial. In the context of acute SAH, Fiorella et al. (4) advised the use of a staged procedure. First, the occlusion of the aneurysm dome is performed with coils. Second, stent-supported coil embolization is performed with a few days interval. In cases where it is not possible, the investigators suggest the administration of 650 mg ASA immediately after coil embolization and commencement of dual antiplatelet therapy the next day (4). Our practice differs. A stent is implanted first, and then coils are packed into an aneurysmal sack through the stent mesh during the same procedure. Patients are administered 150 mg ASA just prior to the procedure of stent placement and after coil packing, dual antiplatelet therapy with 150 mg ASA and 75 mg of clopidogrel is initiated. In the present case, coil embolization after stent placement was initially planned, but was not attempted due to a significantly decreased flow within the sack. The patient was administered 150 mg ASA prior to the procedure and ASA therapy was continued for life. The decision not to administer a second antiaggregative agent after the procedure was made, as the aneurysm dome was unsecured with coils. Additionally, the stent used was short (12 mm) and was not placed through the basilar bifurcation. During a 30-minute observation, blood flow within the stent and the basilar artery was normal without any signs of vasospasm.
Although the use of dual antiplatelet therapy is considered as superior to ASA monotherapy in unruptured aneurysms, the optimal treatment regimen for ruptured aneurysms has to be determined and an optimal treatment regimen is currently being investigated.
There were no thromboembolic events or rebleeding noted and one-month follow-up angiography demonstrated complete thrombosis within the aneurysm. Similar cases of aneurysm thrombosis after stent placement without coil embolization have been described (7). However, thromboembolic complications associated with stent implantation in the acute setting of SAH have been reported (3, 4).
The application of stents have been gaining wide acceptance in cases of wide-necked aneurysms. In some cases, stent placement only may successfully cause total aneurysm occlusion.
Figures and Tables
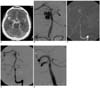
Fig. 1
Obliteration of basilar artery aneurysm in 45-year-old man.
A. Non-enhanced CT scan reveals massive subarachnoid hemorrhage (Fisher grade IV) with intraventricular hemorrhage.
B. Angiogram shows wide-necked, saccular aneurysm on basilar trunk and aplasia of left P1 segment of posterior cerebral artery.
C. Angiographic road map displays stent placement.
D. Angiogram immediately following stent deployment shows significantly decreased intraaneurysmal flow.
E. Follow-up angiogram after one month demonstrates complete occlusion of aneurysm.
References
1. Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991. 75:8–14.
2. Kis B, Weber W, Berlit P, Kuhne D. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo). Neurosurgery. 2006. 58:443–450.
3. Szikora I, Berentei Z, Kulcsar Z, Barath K, Berez A, Bose A, et al. Endovascular treatment of intracranial aneurysms with parent vessel reconstruction using balloon and self expandable stents. Acta Neurochir (Wien). 2006. 148:711–723.
4. Fiorella D, Albuquerque FC, Han P, McDougall CG. Preliminary experience using the Neuroform stent for treatment of cerebral aneurysms. Neurosurgery. 2004. 54:6–16.
5. Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res. 2002. 24:S33–S42.
6. Soeda A, Sakai N, Sakai H, Iihara K, Nagata I. Endovascular treatment of asymptomatic cerebral aneurysms: anatomic and technical factors related to ischemic events and coil stabilization. Neurol Med Chir (Tokyo). 2004. 44:456–465.
7. Vanninen R, Manninen H, Ronkainen A. Broad-based intracranial aneurysms: thrombosis induced by stent placement. AJNR Am J Neuroradiol. 2003. 24:263–266.
8. Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005. 56:E416.
9. Benndorf G, Herbon U, Sollmann WP, Campi A. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. AJNR Am J Neuroradiol. 2001. 22:1844–1848.
10. Yu SC, Zhao JB. A steady flow analysis on the stented and non-stented sidewall aneurysm models. Med Eng Phys. 1999. 21:133–141.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download