Abstract
A benign anastomotic stricture is a common complication of upper gastrointestinal (UGI) surgery and is difficult to manage conservatively. Fluoroscopically guided balloon dilation has a number of advantages and is a safe and effective procedure for the treatment of various benign anastomotic strictures in the UGI tract.
A benign anastomotic stricture is a common complication of upper gastrointestinal (UGI) surgery and is difficult to manage conservatively (1-4). In treatment of the obstructive symptoms caused by benign anastomotic strictures, surgical revision is generally avoided, if possible, because of a high rate of morbidity or mortality (5, 6).
In the field of interventional radiology, fluoroscopically guided balloon dilation (FGBD) has been widely accepted as a safe and effective treatment modality for various types of benign anastomotic strictures of the UGI tract (1-3, 6-11). A benign anastomotic stricture is usually a short lesion resulting from a single and discrete event and is ideally suited to treatment with balloon therapy (11, 12). Therefore, interventional radiologists should be familiar with the technique that varies according to each type of benign anastomotic stricture of the UGI tract. The purpose of this pictorial essay is to review the methods, results, and complications of FGBD for the treatment of various benign anastomotic strictures in the UGI tract.
A 2- or 3-week interval has been suggested as the minimum "safe" time between surgery and dilation of an anastomotic stricture (9, 13). A barium study is usually performed prior to FGBD in order to delineate a stricture and plan the procedure. Local anesthesia (use of an oral spray of lidocaine hydrochloride) or conscious sedation (administration of 1-4 mg of intravenous midazolam hydrochloride) is provided immediately before the procedure.
A small amount of water-soluble contrast medium is swallowed to opacify the stricture. A 0.035-inch angled exchange guide wire is inserted with fluoroscopic guidance through the mouth and across the stricture. A deflated balloon catheter is passed over the guide wire to a position astride the stricture. The deflated balloon is inflated slowly with diluted water-soluble contrast medium until the waist deformity created by the stricture disappears from the balloon contour or if the patient develops severe pain. Elimination of the waist is an indicator of a successful dilation. Balloon inflation is maintained for one minute. If the waist of the balloon is not eliminated during the first inflation, a second or third inflation is performed.
The optimal gastrointestinal luminal diameter achieved by dilation may vary with the location of the lesion, the clinical goal, and the age of the patient (5). A luminal diameter of 20 mm in an anastomotic stricture of the esophagus may allow asymptomatic oral intake (8, 9). In cases of a stricture at gastrojejunostomies, some investigators have targeted a desirable luminal diameter of 15 mm (9, 10). Other investigators have insisted that a luminal diameter of at least 20 mm should be achieved to prevent symptoms of obstruction in benign anastomotic strictures at gastroenterostomy (2). In addition, 20 mm was considered as an optimal diameter to achieve in patients with a benign anastomotic stricture at esophagojejunostomy (1).
Dilations should be performed with increasing balloon diameters starting with small balloons and progressing to larger sizes in order to minimize the rate of perforation. To prevent recurrent symptoms and allow for the fewest complications, 20-25 mm is recommended as the optimal maximum balloon diameter (1, 2, 6, 8). Most often, a 10-15 mm diameter balloon is initially used. If dilation with a 10-15 mm balloon is easily accomplished and the patient tolerates the procedure well, the caliber of the balloon catheter is increased up to 20-25 mm (Fig. 1). If at any point the patient complains of intolerable pain, or if a persistent waist remains in the balloon despite a maximal inflation pressure (i.e., 6 atm), the dilation should be discontinued (10). In pediatric patients with anastomotic strictures after surgical repair of esophageal atresia (3, 14), the first dilation is usually made with a balloon 4-8 mm in diameter. If dilation is easily accomplished, the size of the balloon is gradually increased by the use of 2-mm steps during the same session.
Immediately after dilation, patients swallow a small amount of water-soluble contrast medium for an evaluation of the presence of perforation. If there is no evidence of perforation, a barium study is performed to look for a mucosal tear and to evaluate the degree of barium passage.
After balloon dilation, a follow-up barium study is usually performed at one month after the procedure to verify the status of the stricture and the presence of passage disturbance, and then patients are advised to visit the outpatient clinic if symptoms recur (2, 5, 8).
A successful dilation is defined as relief of the obstructive symptoms and good gastric emptying as demonstrated on a routine follow-up barium study. If the patient symptoms persist or recur, repeat balloon dilation is required.
The use of fluoroscopy allows not only the identification of the proximal and distal ends of a stricture, but also allows visual control of the entire balloon catheter during its placement and inflation (1, 2, 6, 8). The use of fluoroscopy helps to minimize the risk of perforation caused by inappropriate advancement of instruments and misplacement of the balloon during inflation caused by its sliding. Complete visual control is more important when treating anastomotic strictures that occur at an acutely angled site such as a gastroenterostomy. However, it is important for the patient to initially undergo endoscopy in order to confirm the diagnosis and thereby exclude the presence of a possible malignancy.
Anastomotic strictures occur in 3 to 13% of patients after gastric surgery; these strictures can lead to prolonged vomiting and nutritional deficiencies (15). In a previous study, FGBD was clinically effective in 94% (16/17) of patients with a benign anastomotic stricture after Billroth I (gastroduodenostomy) (Fig. 1) or Billroth II (gastrojejunostomy) (Fig. 2) reconstruction for a malignancy or gastric ulcer (mean follow-up period, 14 months) and there were no major complications (2).
Restrictive stomach surgery, i.e. gastric bypass surgery and vertical banded gastroplasty, are routinely performed to create a small gastric reservoir with a restrictive outlet, thereby causing early satiety and decreased food intake in morbidly obese patients. In a study of 24 patients with symptoms of obstruction following gastric surgery for morbid obesity, 50% of the patients experienced relief of symptoms after FGBD (mean follow-up period, 29 months) (6). There were no immediate or late complications of the FGBD procedure in this study.
A benign anastomotic stricture after total gastrectomy is also a good indication for FGBD (Fig. 3). In one study, obstructive symptom relief was maintained (mean asymptomatic period, 23 months) after FGBD in 91% (21/23) of patients with benign strictures at esophagojejunostomy following total gastrectomy, and there were no major complications (1). A minor complication, a small intramural tear, occurred in 4% (1/23) of the patients.
Anastomotic strictures occur in 18-50% of patients undergoing repair of esophageal atresia (3, 14). In one series (14), all 25 children with anastomotic strictures secondary to surgical repair of esophageal atresia, experienced symptom improvement following FGBD over a follow-up period from 4 to 33 months. The only serious complications were two cases of transmural perforation that responded to conservative treatment. In another series (3), FGBD obstructive symptoms were relieved in 93% (27/29) of patients with strictures following surgical repair of esophageal atresia, during a mean follow-up period of 37 months. In this study, transmural perforation occurred in three children; two patients received conservative management and one patient underwent surgery.
Ivor-Lewis esophagectomy is one of the popular procedures for esophageal resection; it includes abdominal incision, right thoracotomy, and esophagogastric anastomosis (16). In a previous study, following FGBD, obstructive symptoms were relieved in 95% (59/62) of patients with benign anastomotic strictures after Ivor-Lewis esophagectomy (Fig. 4) during a mean follow-up period of 28 months, and there were no major complications (8).
Rupture after FGBD for benign anastomotic strictures in the UGI tract is rare (3-5%), and is a mild mucosal tear in all cases (Fig. 5) (1, 8, 13). However, in pediatric patients with anastomotic strictures secondary to surgical repair of esophageal atresia, serious transmural rupture after FGBD occurs in 8-10% of the patients (3, 14). Even if a transmural rupture occurs, conservative management including fasting, antibiotic therapy, and parenteral alimentation in most cases can be used to treat the rupture (3. 14). In cases of transmural ruptures with persistent extraluminal leakage even after conservative treatment, insertion of a covered metallic stent may be effective to prevent leakage. A transmural rupture with progressive clinical deterioration is an absolute surgical indication.
Figures and Tables
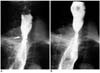
Fig. 1
Fluoroscopically guided balloon dilation for anastomotic stricture at gastroduodenostomy.
A. UGI series before balloon dilation shows anastomotic stricture (arrow) at gastroduodenostomy.
B-E. Anastomotic stricture is initially dilated using 15-mm-diameter balloon. As dilation is easily accomplished, caliber of balloon catheter is increased to 20 mm.
F, G. UGI series immediately (F) and one month after (G) balloon dilation showed improvement of luminal diameter (arrows).
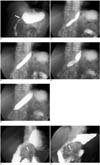
Fig. 2
Fluoroscopically guided balloon dilation for anastomotic stricture at gastrojejunostomy.
A. UGI series before balloon dilation shows severe anastomotic stricture (arrow) at gastrojejunostomy with no passage of contrast medium.
B, C. 20-mm-diameter balloon is placed and is inflated until waist forms by stricture disappeared.
D. One month after balloon dilation, anastomotic stricture is greatly improved (arrows).
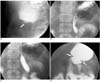
Fig. 3
Fluoroscopically guided balloon dilation for anastomotic stricture at esophagojejunostomy.
A. UGI series before balloon dilation shows severe anastomotic stricture (arrow) at esophagojejunostomy.
B, C. 20-mm-diameter balloon is placed and is inflated until waist forms by stricture disappeared.
D. Immediately after balloon dilation, stricture is greatly improved (arrow).
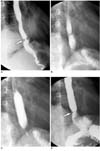
Fig. 4
Fluoroscopically guided balloon dilation for anastomotic stricture after Ivor-Lewis surgery.
A. UGI series before balloon dilation shows anastomotic stricture (arrow) at esophagogastrostomy.
B, C. 20-mm-diameter balloon is placed and is inflated until waist forms by stricture disappeared.
D. Immediately after balloon dilation, stricture is greatly improved (arrow).
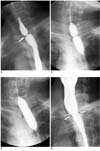
Fig. 5
Intramural rupture of anastomosis (type 1 rupture) after fluoroscopically guided balloon dilation for anastomotic stricture after Ivor-Lewis surgery. UGI series obtained immediately after balloon dilation (A) shows small amount of contrast leakage (arrow). One-month follow-up UGI series (B) shows healed intramural tear.
Acknowledgment
The authors thank Bonnie Hami, MA (USA) for her editorial assistance in the preparation of this manuscript.
References
1. Cho YK, Shin JH, Kim BS, Yook JH, Song HY, Kim JH, et al. Fluoroscopically guided balloon dilation of anastomotic strictures after total gastrectomy: long-term results. AJR Am J Roentgenol. 2007. 188:647–651.
2. Kim JH, Shin JH, Bae JI, Di ZH, Lim JO, Kim TH, et al. Gastric outlet obstruction caused by benign anastomotic stricture: treatment by fluoroscopically guided balloon dilation. J Vasc Interv Radiol. 2005. 16:699–704.
3. Ko HK, Shin JH, Song HY, Kim YJ, Ko GY, Yoon HK, et al. Balloon dilation of anastomotic strictures secondary to surgical repair of esophageal atresia in a pediatric population: long-term results. J Vasc Interv Radiol. 2006. 17:1327–1333.
4. Jha S, Levine MS, Rubesin SE, Dumon K, Kochman ML, Laufer I, et al. Detection of stricture on upper gastrointestinal tract radiographic examinations after laparoscopic Roux-en-Y gastric bypass surgery: importance of projection. AJR Am J Roentgenol. 2006. 186:1090–1093.
5. Kim JH, Shin JH, Di ZH, Ko GY, Yoon HK, Sung KB, et al. Benign duodenal strictures: treatment by means of fluoroscopically guided balloon dilation. J Vasc Interv Radiol. 2005. 16:543–548.
6. Vance PL, de Lange EE, Shaffer HA Jr, Schirmer B. Gastric outlet obstruction following surgery for morbid obesity: efficacy of fluoroscopically guided balloon dilation. Radiology. 2002. 222:70–72.
7. Holt PD, de Lange EE, Shaffer HA Jr. Strictures after gastric surgery: treatment with fluoroscopically guided balloon dilatation. AJR Am J Roentgenol. 1995. 164:895–899.
8. Kim HC, Shin JH, Song HY, Park SI, Ko GY, Yoon HK, et al. Fluoroscopically guided balloon dilation for benign anastomotic stricture after Ivor-Lewis esophagectomy: experience in 62 patients. J Vasc Interv Radiol. 2005. 16:1699–1704.
9. de Lange EE, Shaffer HA Jr. Anastomotic strictures of the upper gastrointestinal tract: results of balloon dilation. Radiology. 1988. 167:45–50.
10. McLean GK, Cooper GS, Hartz WH, Burke DR, Meranze SG. Radiologically guided balloon dilation of gastrointestinal strictures. Part I. Technique and factors influencing procedural success. Radiology. 1987. 165:35–40.
11. McLean GK, Cooper GS, Hartz WH, Burke DR, Meranze SG. Radiologically guided balloon dilation of gastrointestinal strictures. Part II. Results of long-term follow-up. Radiology. 1987. 165:41–43.
12. Weintraub JL, Eubig J. Balloon catheter dilatation of benign esophageal strictures in children. J Vasc Interv Radiol. 2006. 17:831–835.
13. Kim JH, Song HY, Park SW, Yoon CJ, Shin JH, Yook JH, et al. Early symptomatic strictures after gastric surgery: palliation with balloon dilation and stent placement. J Vasc Interv Radiol. 2008. 19:565–570.
14. Said M, Mekki M, Golli M, Memmi F, Hafsa C, Braham R, et al. Balloon dilatation of anastomotic strictures secondary to surgical repair of oesophageal atresia. Br J Radiol. 2003. 76:26–31.
15. Ahmad J, Martin J, Ikramuddin S, Schauer P, Slivka A. Endoscopic balloon dilation of gastroenteric anastomotic stricture after laparoscopic gastric bypass. Endoscopy. 2003. 35:725–728.
16. Bains MS. Ivor Lewis esophagectomy. Chest Surg Clin N Am. 1995. 5:515–526.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download