Abstract
Objective
To evaluate the early clinical experience associated with radiofrequency (RF) ablation in patients with renal cell carcinoma (RCC).
Materials and Methods
The RF ablation treatment was performed on 17 tumors from 16 patients (mean age, 60.5 years; range, 43-73 years) with RCC. The treatment indications were localized, solid renal mass, comorbidities, high operation risk, and refusal to perform surgery. All tumors were treated by a percutaneous CT (n = 10), followed by an US-guided (n = 2), laparoscopy-assisted US (n = 2), and an open (n = 2) RF ablation. Furthermore, patients underwent a follow-up CT at one day, one week, one month, three and six months, and then every six months from the onset of treatment. We evaluated the technical success, technical effectiveness, ablation zone, benign periablation enhancement, irregular peripheral enhancement, and complications.
Results
All 17 exophytic tumors (mean size, 2.2 cm; range, 1.1-5.0 cm) were completely ablated. Technical success and effectiveness was achieved in all cases and the mean follow-up period was 23.8 months (range, 17-33 months). A local recurrence was not detected in any of the cases; however, five patients developed complications as a result of treatment, including hematuria (n = 2), mild thermal injury of the psoas muscle (n = 1), mild hydronephrosis (n = 1), and fistula formation (n = 1).
Renal cell carcinoma (RCC) is the third most common genitourinary tumor, accounting for 2% of all adult cancers (1). The incidence of RCC continues to increase with over 30,000 new cases recorded in the United States for the year 2000 (1). This increase is associated with the advent of modern imaging techniques such as ultrasonography and CT, which can detect very early clinical stage small and localized renal tumors. Previous studies have found that kidney tumors measuring less than 3 cm in diameter are generally not associated with metastasis (2). At the same time, recent advances have led to the use of nephron-sparing surgery techniques, such as a partial nephrectomy or laparoscopic nephrectomy in selected patients with small renal tumors (3, 4). Some patients, however, are not ideal candidates for renal tumor resections because of comorbidities, limited renal functional reserve, or other complicating factors (5). Therefore, for patients that are unsuitable for surgical interventions or with small tumors, minimally invasive therapies capable of destroying tumors without damaging renal functions would be particularly useful (6).
Radiofrequency (RF) ablation is a thermal ablation method that uses high frequency energy to induce cell death. The conduction of the RF causes molecular friction and heat. In turn, this heat energy induces tissue necrosis as a result of denaturation and coagulation. RF ablation has been utilized in early clinical trials for the treatment of hepatocellular carcinoma, hepatic and cerebral metastasis, as well as benign bone tumors such as osteoid osteomas (7-10). In 1998, a case report was published describing the use of a percutaneous RF ablation under ultrasound guidance as the treatment of choice for RCC in human patients (11). It is anticipated that RF ablation will provide a minimally invasive technique for treating small renal tumors. The purpose of this study is to evaluate our early experience with patients who underwent RF ablations for treatable localized renal tumors, retrospectively.
Between August 2004 and December 2005, 17 RF ablations were performed on 16 patients (4 females and 12 males) with renal tumors. The mean patient age was 60.5 years (range, 43-73 years) and the diagnosis of RCCs was based on the fine needle biopsy results in eight renal tumors. The nine remaining renal tumors were treated on the basis of CT results that were consistent with RCC. Indications for RF ablations included coexistent morbidities (n = 5), high surgical or anesthetic risks (n = 6), refusal to undergo surgery (n = 3), and the presence of bilateral RCCs (n = 2). The location of the renal tumors were classified as exophytic, central, or mixed based on their location (12, 13). Prothrombin time, partial thromboplastin time, complete blood count, and serum creatinine level were assessed before and after RF ablation in all cases. Informed consent was obtained for the procedure in all cases. The Institutional Review Board was not required for retrospective studies at Dong-A University. The patient characteristics are summarized in Table 1.
A percutaneous CT (n = 10) or US-guided (n = 2) RF ablation was performed in 12 of 16 patients. An RF ablation was performed under laparoscopy-assisted US guidance (n = 2) and the open approach (n = 2) with the remaining patients. A percutaneous RF ablation was performed under intravenous sedation with 2-4 mg of midazolam hydrochloride (Roche, Fontenaysous-Bois, France) and 100-300 µg of fentanyl citrate (Hana Pharm. Co., Hwasung, Korea). Local analgesia with 2% lidocaine (Huons, Hwaseong, Korea) was administered in combination with intravenous analgesia. The patients were placed in a prone or modified lateral position, according to the tumor location. An open or laparoscopic approach was inducted by general anesthesia using 100-300 mg of propofol (Dongkook Pharm Co., Jincheon, Korea) in four patients. All patients required hospital admission after the RF ablation for close observation.
An RF ablation was performed with a 200 W generator (Radionics, Burlington, MA) using single (with one 2.0-3.0 cm tip) internally cooled electrodes (Radionics, Burlington, MA) with impedance-controlled pulsed current. The length of the exposed tip was chosen by the operator based on tumor size and location. The active treatment time was 12 minutes, as per the manufacturer's recommendations. Based on the size and location of the lesion, overlapping ablations were performed by repositioning the electrode to ablate the entire tumor. A session was defined as one visit at the radiology department, in which one or more RF applications were performed.
All patients underwent a contrast-enhanced CT before and after an RF ablation. A follow-up CT was performed at one day, one week, one month, three and six months, and then every six months thereafter. In addition, a follow-up CT was performed at one day and one week to assess the technical success and immediate or periprocedural complication. A one month follow-up CT was performed to determine whether additional ablation treatment was required. In addition, a follow-up CT was performed after three and six months, and then every six months to detect any new areas of enhancement or an increase in lesion size in ablated tumor region. The treated tumors were assessed for technical success, technical effectiveness, ablation zone, benign periablation enhancement, irregular peripheral enhancement, and complications upon a follow-up CT.
Tumors that were treated to the full extent according to the protocol, which was assessed at the time of the procedure, were considered to be technically successful. Technical effectiveness refers to a prospectively defined point in time at which "complete ablation" of the macroscopic tumor, confirmed by follow-up imaging, was achieved. The technical effectiveness rate was defined as the percentage of tumors that were successfully eradicated following the initial procedure or additional sessions within a one month follow-up period.
The ablation zone was defined by measuring the size change of the minimal contrast enhancement (i.e. < 20 HU for CT) observed by radiological imaging soon after the ablation. The benign periablation enhancement was defined as a thin, concentric, and uniform rim peripheral enhancement with smooth inner margins on a contrast-enhanced CT. On the other hand, an irregular peripheral enhancement was defined as a scattered, nodular, or eccentric peripheral enhancement on a follow-up CT.
We analyzed the rate of complication based on the following time categories following the ablation: 6-24 hours, periprocedural complications (within 30), and delayed complications (greater than 30 days).
The treatment data for each patient are summarized in Table 2. All the 17 renal tumors observed in the 16 patients were subjected to an RF ablation treatment. The mean tumor size was 2.2 cm (range, 1.6-5.0 cm), and all tumors were exophytic. The mean CT follow-up period was 23.8 months (range, 17-33 months). Over the radiologic follow-up period, a total of 13 tumors in 12 patients were successfully treated in one ablation session (Fig. 1), and four tumors from four patients required more than one session based upon follow-up CT scan results. One tumor required 3 sessions, as a result of persistent enhancement within the tumor. Also, seven tumors from seven patients required electrode repositions since the tumor size was too large. Technical success and technical effectiveness rates were achieved in all cases (100%) and the mean total procedure time was 97.8 minutes (range, 32-260 minutes). However, except for the open approach, the mean procedure time was 83.6 minutes.
The postablation serum creatinine levels were analyzed, and revealed that one patient (patient 6) showed an increase in creatinine levels (from 1.2 to 1.5 mg/dl); however, levels returned to a normal range after seven days.
Upon a follow-up CT, all the tumors revealed a variable degree of size reduction (mean diameter, 0.6 cm; range, 0.2-1.7 cm) compared to the pretreatment CT. Irregular peripheral enhancement, indicating incomplete ablation, was noted in four tumors from the one day follow-up CT. As a result, additional procedure sessions were performed. A benign periablation enhancement was observed in eight tumors from seven patients, which disappeared within three months in all tumors (Fig. 2). No tumors were detected in our patients developed metastases during the follow-up period.
Five patients (31%) experienced postablation complications, with immediate complications noted in three patients. Two patients developed gross hematuria after a single ablation, which was resolved within four days. In one patient, a mild thermal injury of the psoas muscle was noted in patients with tumors abutting these areas after the second session. Periprocedural or delayed complications were reported in two patients. In addition, a mild case of hydronephrosis was noted in one patient with a 1.6 cm sized tumor located in the anterior lower pole of the right kidney and has been continued on a follow-up CT (Fig. 3). Moreover, another patient had a small amount of contrast leakage noted from a 12 month follow-up CT, which had a suspected fistula formation. These two patient's creatinine levels were below normal range and no symptoms were noted in the follow-up CT period. Furthermore, no patients required a blood transfusion, intensive care or additional therapy.
Management options for RCCs continue to evolve, with the most recent developments in the areas of nephron-sparing and laparoscopic procedures (3, 4). The trend toward less invasive RCC treatments is at least partly due to the increasing number of RCCs diagnosed today. A percutaneous RF ablation of malignant tumors has been developed as a feasible option for patients with primary and metastatic hepatic lesions that are not good candidates for traditional surgery (7, 8). This approach may have a role in the management of renal tumors. One report in the long-term data following an RF ablation concluded that the RF ablation technique was the most successful treatment of small exophytic renal tumors (12).
Tumor size is an important predictor of successful treatment in RF ablation of renal tumors. Despite advances in electrode design, the successful ablation of tumors, greater than 4 cm in diameter, has been a challenge (14). Therefore, smaller renal tumors are ideal candidates for an RF ablation. On the other hand, the larger tumors require multiple overlapping ablations and, in some cases, return visits for additional ablation sessions (12, 13).
The location of the tumor within the kidney may also play an important role in the efficacy of the RF ablation treatment for renal masses, and may influence the success of an ablation. A central tumor ablation fails more frequently because of a heat sink effect, in which a regional vascular flow reduces the extent of the thermally induced coagulation (15). By contrast, exophytic lesions are surrounded by perirenal fat, which serves as a heat insulator and allows the achievement of higher temperatures during RF ablation. This phenomenon was first described in liver tumors and referred to as the "oven effect" where hepatic tumors surrounded by a fibrotic capsule and surrounding cirrhotic liver tissue are more easily treated (16). As a result of this phenomenon, exophytic renal tumors have an increased likelihood of successful ablation. Therefore, size and location are good predictors of success, with small exophytic renal tumors being the most suitable candidates for RF ablation (12, 13, 17-19). These considerations led us to perform an RF ablation for exophytic or peripherally located tumors only. All 17 tumors up to 5 cm in diameter (small or intermediate tumors) were successfully treated after the completion of the procedures.
We usually performed an RF ablation using a percutaneous CT guidance. At the beginning of the RF ablation, a percutaneous US-guided approach was performed in two patients. However, we preferred the CT guidance because the electrode was more reliably placed, and the production of gas at the ablation site did not obscure the lesion for additional treatments. Consequently, an overlapping ablation was easily performed by repositioning the electrode.
For patients with difficult access points (patients with multiple lesions in a solitary kidney, an intervening lung parenchyma, bowel, or thin patients with an anterior lesion) or comorbidities, a laparoscopy-assisted US guidance or open intraoperative RF ablations should be considered as a treatment option. Among all of the study cases, the open intraoperative RF ablation was carried out in two patients, in which, one operation was performed as a result of esophageal cancer with RF ablation. In addition, two patients were treated with a laparoscopy-assisted US guided RF ablation to isolate the targeted tumors away from adjacent the normal structure, such as the colon or psoas muscle.
We evaluated follow-up images using the criteria reported by Goldberg et al. (6) and assessed the treated RCCs for lesion size as well as benign periablation enhancement and irregular peripheral enhancement. A benign periablation enhancement typically suggests a benign physiologic response to a thermal injury (initially: reactive hyperemia, subsequently: fibrosis and giant cell reaction). This transient finding, measuring up to 1-2 mm can be observed immediately after ablation, and can last for up to 3 months after the ablation. An irregular peripheral enhancement represents a residual tumor that may occur at the treatment margin. This lesion grows in a scattered, nodular, or eccentric pattern, which indicates an incomplete local treatment (i.e. residual unablated tumor) (6). In this study, a benign periablation enhancement was identified in eight tumors from seven patients and the mean duration of its existence was 20.2 days (range, 1 week 3 months). An irregular peripheral enhancement was noted in four tumors, followed by additional procedure sessions.
An RF ablation can cause various complications. Major complications, include hemorrhaging requiring a transfusion, ureteral strictures, tumor seeding in the electrode track, and urine leakage. Minor complications in the RF ablation include self-limited paresthesias and transient hematuria (12, 17). In our study, five complications were encountered; however, all patients were successfully treated without long-term adverse effects. The patient diagnosed with a mild case of hydronephrosis, after having undergone an RF ablation, was discovered to have a tumor located just adjacent to the renal pelvis and was managed by supportive care without any intensive interventions. However, the patient required a longer hospital admission than the other patients enrolled in the study. One patient was temporarily diagnosed with a moderate elevation in serum creatinine levels following an RF ablation; however, the patient's creatinine levels returned to within a normal range after seven days. It is presumed that acute renal failure occurred as a result of the renal hypoperfusion during the laparoscopic procedure. The results of this study suggest that image-guided RF ablation of small localized renal tumors is a safe procedure with an extremely low major complication rate.
Despite the findings, this study had several limitations, including patient sample size and the relatively short follow-up period (23.8 months). Therefore, a long-term radiographic and clinical follow-up would be required to assess the oncologic outcome of RF ablations. In addition, the histopathologic diagnosis was not confirmed in nine renal tumors. However, over 90% of the solid renal masses are RCC. Lastly, imaging follow-ups were only performed with contrast-enhanced CT scans to determine the extent of an "unablated residual tumor".
In conclusion, the RF ablation is a minimally invasive procedure that appears to be a safe and effective treatment for small localized exophytic renal tumors. Those lesions not amenable to a percutaneous RF ablation could be treated with laparoscopy-assisted US-guided or open intraoperative RF ablation. Although, the mean follow-up period in this study was relatively short, our early experience with RF ablation demonstrates that it is a safe and effective treatment for small exophytic renal tumors in a selected group of patients.
Figures and Tables
Fig. 1
Well ablated, small exophytic renal cell carainoma in 56-year-old woman who underwent RF ablation.
A. Contrast-enhanced CT scan before RF ablation demonstrates solid enhancing exophytic renal tumor located at lower polar region of left kidney (arrow).
B. RF ablation of tumor was performed under CT guidance. Unenhanced CT scan during treatment demonstrates electrode within renal tumor (arrow).
C. One-month follow-up contrast-enhanced CT scan demonstrates no periablation enhancement or residual contrast enhancement within tumor bed (arrow), indicating technical success.
D. Two-year follow-up contrast-enhanced CT scan demonstrates no areas of abnormal contrast enhancement within tumor and shows further decrease in size of renal tumor (arrow).
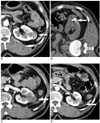
Fig. 2
Small exophytic renal cell carcinoma in 45-year-old woman.
A. Contrast-enhanced CT before RF ablation demonstrates enhancing exophytic renal tumor located at lower polar region of right kidney (arrow).
B. One-day follow-up contrast-enhanced CT scan demonstrates peripheral, curvilinear enhancement (arrow) in ablated tumor, indicating possible reactive hyperemia or remnant tumor.
C. One-month follow-up contrast-enhanced CT scan demonstrates no previous peripheral enhancement (arrow) and any residual contrast enhancement within tumor, suggesting benign periablation enhancement.
D. Two-year follow-up contrast-enhanced CT scan demonstrates no evidence of enhancement within ablation zone (arrow).
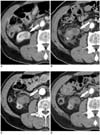
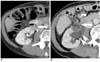
Fig. 3
Development of mild hydronephrosis in 73-year-old male patient who underwent RF ablation for renal cell carcinoma.
A. Contrast-enhanced CT scan before RF ablation reveals 1.6 cm solid enhancing exophytic renal tumor located at lower polar region of right kidney
(arrow).
B. Three-month follow-up contrast-enhanced CT scan shows no remnant contrast enhancement (arrow) in tumor but reveals mild hydronephrosis (arrowheads) in right kidney. Patient did not require interventional procedure for hydronephrosis.
References
1. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000. 50:7–33.
2. Rendon RA, Stanietzky N, Panzarella T, Robinette M, Klotz LH, Thurston W, et al. The natural history of small renal masses. J Urol. 2000. 164:1143–1147.
3. Duque JL, Loughlin KR, O'Leary MP, Kumar S, Richie JP. Partial nephrectomy: alternative treatment for selected patients with renal cell carcinoma. Urology. 1998. 52:584–590.
4. Nakada SY, McDougall EM, Clayman RV. Laparoscopic extirpation of renal cell cancer: feasibility, questions, and concerns. Semin Surg Oncol. 1996. 12:100–112.
5. Zagoria RJ. Imaging-guided radiofrequency ablation of renal masses. Radiographics. 2004. 24:S59–S71.
6. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005. 235:728–739.
7. Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998. 170:1015–1022.
8. Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, et al. Hepatic metastases: percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology. 1997. 205:367–373.
9. Anzai Y, Lufkin R, DeSalles A, Hamilton DR, Farahani K, Black KL. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol. 1995. 16:39–48.
10. Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol. 2001. 12:717–722.
11. McGovern FJ, Wood BJ, Goldberg SN, Mueller PR. Radiofrequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol. 1999. 161:599–600.
12. Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003. 226:417–424.
13. Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000. 217:665–672.
14. Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996. 3:636–644.
15. Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998. 9:101–111.
16. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.
17. Farrell MA, Charboneau WJ, DiMarco DS, Chow GK, Zincke H, Callstrom MR, et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol. 2003. 180:1509–1513.
18. Schiller JD, Gervais DA, Mueller PR. Radiofrequency ablation of renal cell carcinoma. Abdom Imaging. 2005. 30:442–450.
19. Park S, Anderson JK, Matsumoto ED, Lotan Y, Josephs S, Cadeddu JA. Radiofrequency ablation of renal tumors: intermediate-term results. J Endourol. 2006. 20:569–573.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download