Abstract
Objective
Ultrasound-guided needle localization has been used prior to the surgical excision of nonpalpable breast lesions. The aim of the study was to assess the feasibility of the use of a saline immersion specimen ultrasound technique (immersion-US) to confirm the successful removal of breast lesions.
Materials and Methods
The devised immersion-US technique was used to examine the excised tissues of 72 ultrasound-guided needle localized breast lesions of 58 patients (34 benign lesions, 30 high-risk lesions and 8 malignant lesions). Freshly excised specimens were placed in a container filled with saline and one radiologist scanned the surgically excised specimens using a high-frequency linear transducer. We evaluated successful lesion removal and the qualities of the immersion-US images. Miss rates were determined by the use of postoperative ultrasound during follow-up.
Results
All 72 lesions were identified by the use of immersion-US and satisfactory or excellent quality images were obtained for most lesions (70/72, 97%). Five (7%) lesions were initially identified as incompletely excised, based on the immersion-US findings, and prompt re-excision was undertaken. Follow-up ultrasound examinations showed no residual mass in the surgical field in any patient.
The increased popularity of breast screening programs has resulted in an increased detection rate of nonpalpable breast lesions. Mammography and breast ultrasonography (US) are effective to detect and to diagnose such lesions, and preoperative breast US facilitates performing of a biopsy and needle placement in nonpalpable breast lesions. Moreover, for surgical excision of nonpalpable breast lesions, the lesions should be localized before surgery and specimen imaging should be performed to ensure complete removal.
Mammography has been widely used as an imaging modality for needle localization of nonpalpable breast lesions, especially calcifications, and specimen radiography has been traditionally used for standard specimen imaging. However, the number of detected nonpalpable breast lesions continues to increase due to the use of breast US, and some of these lesions seen on breast US are not visualized by mammography. Thus, for such lesions, localization should be attempted only under US guidance. In addition, the use of breast US has advantages for needle localization as its use reduces the time involved and patient discomfort.
It may sometimes be difficult to identify a lesion in subsequent specimen US images as a specimen may be too small for adequate imaging when a linear transducer is used in air. In addition, needles may become displaced; the excision failure rate associated with needle localization has been reported to be 5-22% (1). Therefore, a prompt, precise, and convenient technique for specimen US is needed to reduce the frequency of missed lesions, and to provide surgeons with confirmation that a nonpalpable breast lesion has been completely excised.
This prospective study was undertaken to assess the feasibility of the use of a saline immersion specimen US technique (immersion-US) to confirm the successful removal of nonpalpable breast lesions after US-guided needle localization.
Institutional review board at the Korea University Ansan Hospital approval and full informed consent for the procedure from the patients were obtained. From September 2005 to October 2007, US-guided needle localization and specimen US were performed for 72 breast lesions in 58 patients. All patients were female, and ages ranged from 22 to 58 years (mean age, 40 years). Five of the 58 patients had a nipple discharge (bloody discharge in three patients and a yellow discharge in two patients). The remaining 53 patients had no clinical symptoms. Preoperative mammographic data was available for 58 lesions and US images for all 72 breast lesions. The mammographic and US findings are shown in Table 1. Radiological findings were evaluated according to the Breast Imaging Reporting and Data System (BI-RADS®) atlas (2). Breast parenchymal patterns by mammography were extremely dense in 18 lesions (31%), heterogeneously dense in 22 lesions (38%), scattered fibroglandular tissues in 12 lesions (21%), and almost fatty in six lesions (10%).
In 43 (60%) of the 72 lesions, tissue sampling was performed before needle localization and surgical excision. An US-guided core needle biopsy was performed for 39 lesions and US-guided fine needle aspiration was performed for four lesions. At our hospital, indications for surgical excision after a core needle biopsy or fine needle aspiration were the following: a lesion with a high risk of malignancy based on a pathological examination (n = 17), a lesion with discordant radiological and pathological findings (n = 10), or surgeon or patient preference (n = 16). High risk lesions for malignancy included a radical scar, atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ, a papillary lesion, or sclerosing adenosis. The remaining 29 (40%) lesions underwent surgical excision with US-guided needle localization without previous tissue sampling because of the following reasons: more than a moderate probability of malignancy based on radiological findings (n = 16), a bloody nipple discharge (n = 3), or surgeon or patient preference (n = 10).
We used an iU 22 unit (Philips Medical Systems, Bothell, WA) with a broad-bandwidth (14-5 MHz) and a linear scanhead for the US examinations, and hook localizer wires (Accura BLN, Medical Device Technologies, Gainesville, FL). Needle localization was performed on the day of surgery by an experienced breast radiologist as follows. Initially, the breast was cleaned with disinfectant, and the transducer head was then coated with a gel and covered with a sterile sheath. A sterile gel was used during the localization procedure. A fenestrated drape was placed on the defined sterile field. Using a free-hand technique, the needle was advanced through the lesion under realtime US guidance in the transverse section and then the needle sheath was removed. After inserting the needle into the lesion, an 'X' was marked on the overlying skin.
After surgical excision, the excised specimen was immediately examined by a radiologist. The mean time between the surgical excision and the US examination was 6.4 minutes (range, 4-8 minutes). Durations of specimen examinations were recorded and the mean time was determined as 5.2 minutes (range, 3-7 minutes). Briefly, a fresh specimen was placed in a plastic container filled with saline. The specimen was held with the left hand leaving the right hand free for scanning (Fig. 1). Specimens were scanned by applying gel to the transducer and placing the transducer on the specimen. When a lesion was found, it was scanned transversely and longitudinally. The breast radiologist then evaluated the accuracy of excision and reported the findings to surgeons in the operating room. If the mass appeared to be complete in the specimen by US, surgery was concluded, but if the lesion was found as incomplete or was located too close to an excision margin (< 0.1 cm), a second excision in the appropriate area was undertaken immediately. Subsequent surgical samples were also subjected to immersion-US.
For four lesions with calcifications as depicted on mammography, specimen radiography was performed in addition to immersion-US. All four of these lesions were identified by specimen radiography and immersion-US. After examining the excised specimens, all specimens were forwarded to the Department of Pathology for a histopathological examination.
We evaluated the qualities of the images of specimen US and assessed the miss rate. Image qualities were categorized as grade 1 (poor), grade 2 (satisfactory), or grade 3 (excellent) by consensus between two radiologists. The US miss rate was determined based on postoperative follow-up imaging findings. Follow-up breast US was performed on 70 (97%) of the 72 lesions. The times between follow-up and the US evaluations of surgical specimen ranged from 18 to 370 days (mean time, 128 days).
On pathological examinations, the specimen sizes ranged from 10 mm to 53 mm (mean size, 31 mm), and the lesions ranged in size from 3 mm to 25 mm (mean size, 12 mm). The pathological diagnoses of the 72 lesions were a benign lesion in 34 cases (47%) (Fig. 2), a high-risk lesion in 30 cases (42%) (Fig. 3) and a malignant lesion in eight cases (11%) (Figs. 4, 5) (Table 2). In the eight malignant lesions and nine high-risk lesions, re-excision was performed after US-guided localization with focal excision, and follow-up US images were obtained before re-excision. There was no residual lesion seen at an excision site on a follow-up US examination for these 17 lesions. All eight malignant lesions were treated surgically with the use of a modified radical mastectomy in four cases and with breast conserving surgery in four cases. Four of the eight malignant were identified as residual carcinomas by a pathological examination after surgery. These four malignant lesions with residual carcinomas were further characterized as three ductal carcinomas in situ and one invasive carcinoma, with sizes ranging from 3 to 9 mm (mean size, 5 mm). Eleven high-risk lesions (seven atypical ductal hyperplasic lesions, two radial scars and two intraductal papillomas) that underwent re-excision showed no residual lesions as seen on US or pathological examinations during follow-up.
All 72 lesions were identified by US in the excised specimens. Five (7%) of the 72 lesions were deemed to have been incompletely excised by immersion-US and prompt re-excision was undertaken. A pathological examination was performed with both the initial and re-excised specimens. In two of these five lesions, the initial specimens were benign lesions; however, high-risk lesions were found on the re-excised specimens (one atypical ductal hyperplasia and one sclerosing adenosis).
In terms of image qualities, two (3%) lesions were assessed as grade 1, six (8%) lesions were assessed as grade 2, and 64 (89%) lesions were assessed as grade 3. Satisfactory or excellent images were obtained for the majority of lesions (70/72, 97%).
On postoperative follow-up breast US examinations, there was no residual mass in the surgical field seen in any patient. Thus, the miss rate was 0%.
Precise localization under imaging guidance is necessary before excising nonpalpable breast lesions. Mammography has been widely used as an imaging-guide for needle localization of nonpalpable breast lesions. However, when breast lesions are identified by preoperative US, US-guided needle localization is desirable. Furthermore, when a breast lesion is detected by mammography and US, US is the preferred modality for imaging guidance as the use of breast US has advantages over mammography in terms of needle localization or skin marking (3-5). For example, US-guided needle localization allows the needle to be observed in real time and allows insertion of the needle directly through the lesion. Moreover, US-guided needle localization is faster; mammography-guided needle localization requires two pairs of check mammograms and the overall procedure for US-guided needle localization rarely lasts less than 20 minutes, even if digital mammography or a stereotactic device is used. In addition, the distance between the skin and a breast lesion is usually less for US-guided needle localization than for mammography-guided needle localization. Therefore, US-guided needle localization can be performed more rapidly and produce better cosmetic results (4, 5).
After US-guided needle localization, US of the specimen is needed to verify successful excision. However, US of specimens is not widely used and few reports exist on the topic (4-7). Nevertheless, the studies performed have demonstrated that US of the specimens is effective to identify the presence of a lesion within a specimen. In previous studies, specimen US of specimens was performed in the operating room, and radiologists wore aseptic dress and had to wait until the procedure was required. However, in the present study, we performed US in an ultrasound room in the radiology department. Fortunately, the operating room and the ultrasound room at the Korea University Ansan Hospital are relatively close, and thus, delivery times averaged only 6.4 minutes.
Studies by Fornage et al. (5) and Feld et al. (8) used saline to perform US of specimens as in the present study. However, in the study by Fornage et al. (5), a relatively small number of cases (18 cases) were evaluated. Moreover, the study by Feld et al. (8) included palpable and nonpalpable breast masses. In the present study, we included 72 nonpalpable breast lesions, which was a sufficient number to assess the feasibility of the use of immersion-US.
For US of specimens, the devised immersion-US technique offers advantages versus the in-air conventional US technique in terms of image quality and efficiency. First, it allows better quality US images to be obtained. The immersion technique involves the use of saline, which fills entrapped air pockets between the transducer and specimen, and thus improves image quality. In the present study, satisfactory or excellent images were obtained for 97% of the lesions, and good quality images were obtained for small and superficial lesions. Furthermore, in terms of decision-making for complete excision, we believe that immersion-US is a better method to utilize than surgical bed US examinations as the image qualities are superior and the procedure times are shorter. Surgeons can perform surgical bed US after excision in the operating room; however, surgical disruptions of soft tissue planes, edema, gas, and hemorrhage are all likely to reduce image quality, and under such circumstances, even an expert radiologist can overlook the presence of a residual lesion. The mean time required for surgical bed US found was reported by Feld et al. (8) as 24.2 minutes. The mean time required for immersion-US in the present study was only 5.2 minutes. A second reason for a preference of immersion-US over conventional in-air specimen US is that immersion-US is performed easily and quickly. Furthermore, radiologists do not have to wear aseptic dress or require the need for special tools. In the present study, we used a small plastic container filled with saline and preformed examinations in 5.2 minutes as opposed to the 22 minutes required by Feld and colleagues (8) for the conventional in-air technique.
In the present study, the miss rate was determined by postoperative US imaging. Follow-up postoperative US images were obtained for all 72 lesions, and no residual lesion was found in any case. Initially, five (7%) of these lesions had been incompletely excised as determined by immersion-US and these lesions were re-excised and reexamined. In two of these five lesions, initial specimens were benign lesions; however, high-risk lesions were found on the re-excised specimens. Thus, if immersion-US had not been performed, surgeons might have missed or underdiagnosed these five breast lesions. The failure rate of excision with US needle localization has been reported as 5-22% (1). Localizing needles are usually inserted at an angle and at considerable distances from lesions, which can cause confusion regarding precise lesion locations. In particular, when lesions are small, a radiologist may fail to place a needle within a lesion. Furthermore, if the localizing needle moves or is not properly placed through a lesion, there is a concern that the surgeon will be unable to locate the lesion without an imaging device during surgery. Therefore, we recommend that immersion-US be conducted after the excision of nonpalpable breast lesions to prevent surgical misses.
To assess tumor margins in malignant breast lesions, a study by Mesurolle et al. (6) showed that the use of US of specimens is limited by false negative and false positive results. In that study, US of specimens had a 21% false positive rate and 23% false negative rate for the assessment of tumor margins in malignant lesions based on the pathological findings. Our results indicated that 11 high-risk lesions (seven atypical ductal hyperplasia, two radial scars and two intraductal papillomas) showed no residual lesion on a pathological examination after second wide excision. However, four (50%) of eight malignant lesions had residual carcinomas as determined on a pathological examination after second surgical excision. Three (75%) of these four malignant lesions with residual carcinomas were ductal carcinomas in situ. Silverstein et al. (9) have noted that 45% of cases of ductal carcinoma in situ that were considered as adequately excised had residual disease, as determined either by re-excision or by mastectomy. Moreover, the thicknesses of free margins were found to be directly related to local recurrence-free survival. Therefore, although a malignant breast lesion may have been deemed as successfully excised based on US of the specimen after needle localization, a second wide excision should be performed to achieve a safe free margin.
In conclusion, US of specimens is required after the surgical excision of US-guided needle localized nonpalpable breast lesions to assess surgical success. In this context, the described immersion technique was demonstrated as a straightforward and effective method. In particular, immersion-US also provided good quality US images of small and superficial breast lesions.
Figures and Tables
 | Fig. 1Description of devised saline immersion technique for specimen US (immersion-US). Specimen is placed in plastic container filled with saline. With one hand holding specimen (A), sample is scanned with linear transducer with other hand (B). |
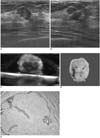 | Fig. 236-year-old woman with fibroadenoma.
A. US image shows 14 mm-sized, angular marginated, oval, hypoechoic mass (arrows).
B. US-guided needle localization was performed for excision, and needle was directly passed through mass (arrowheads).
C. After excision, immersion-US was performed and mass (arrows) was found to be successfully removed.
D. Gross pathological examination shows whitish gray multilobulated solid mass (arrows).
E. Microscopic pathological examination shows well-defined mass with fibrous stroma and elongated tubules (Hematoxylin & Eosin staining; original magnification, ×100).
|
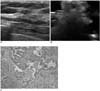 | Fig. 344-year-old woman with intraductal papilloma.
A. US image demonstrates 6-mm sized complex echoic mass (arrows).
B. Immersion-US image shows complete mass excision (arrows).
C. Microscopic pathologic examination demonstrates ductal epithelial papillary projections (Hematoxylin & Eosin staining; original magnification, ×200).
|
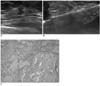 | Fig. 446-year-old woman with ductal carcinoma in situ.
A. US image shows 11-mm sized, spiculated irregularly shaped, isoechoic mass (arrows).
B. Immersion-US image demonstrates successful mass excision (arrows).
C. Microscopic pathological examination demonstrates cribriform and solid type, low-grade ductal carcinoma in situ (Hematoxylin & Eosin staining; original magnification, ×200).
|
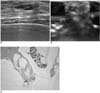 | Fig. 526-year-old woman with ductal carcinoma in situ and mucocele-like tumor.
A. US shows 13-mm sized complex echoic mass (arrows).
B. Immersion-US image demonstrates successful mass excision (arrows).
C. Microscopic pathological examination demonstrates mucin pool with floating mucin producing malignant cells (arrows) (Hematoxylin & Eosin staining; original magnification, ×100).
|
References
1. Snider HC Jr, Morrison DG. Intraoperative ultrasound localization of nonpalpable breast lesions. Ann Surg Oncol. 1999. 6:308–314.
2. American College of Radiology. BI-RADS Committee. . ACR BI-RADS® breast imaging and reporting data system: breast imaging atlas. 2003. Reston, VA: American College of Radiology.
3. Ko K, Han BK, Jang KM, Choe YH, Shin JH, Yang JH, et al. The value of ultrasound-guided tattooing localization of nonpalpable breast lesions. Korean J Radiol. 2007. 8:295–301.
4. Laing FC, Jeffrey RB, Minagi H. Ultrasound localization of occult breast lesions. Radiology. 1984. 151:795–796.
5. Fornage BD, Ross MI, Singletary SE, Paulus DD. Localization of impalpable breast masses: value of sonography in the operating room and scanning of excised specimens. AJR Am J Roentgenol. 1994. 163:569–573.
6. Mesurolle B, El-Khoury M, Hori D, Phancao JP, Kary S, Kao E, et al. Sonography of postexcision specimens of nonpalpable breast lesions: value, limitations, and description of a method. AJR Am J Roentgenol. 2006. 186:1014–1024.
7. Harlow SP, Krag DN, Ames SE, Weaver DL. Intraoperative ultrasound localization to guide surgical excision of nonpalpable breast carcinoma. J Am Coll Surg. 1999. 189:241–246.
8. Feld RI, Rosenberg AL, Nazarian LN, Needleman L, Lev-Toaff AS, Segal SR, et al. Intraoperative sonographic localization of breast masses. J Ultrasound Med. 2001. 20:959–966.
9. Silverstein MJ, Lagios MD, Groshen S, Waisman JR, Lewinsky BS, Martino S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999. 340:1455–1461.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download