Abstract
Objective
The aim of this study is to investigate the effects of cooperative training on the pretreatment assessment of the feasibility to perform Ultrasonography (US) guided percutaneous radiofrequency ablation for patients afflicted with hepatocellular carcinoma.
Materials and Methods
In our prospective study, 146 patients with 200 hepatocellular carcinomas were referred for radiofrequency ablation after triage by hepatologists. Three radiologists with different levels of experience performed the planning US before (group I) and after (group II) cooperative training, to evaluate whether radiofrequency ablation was feasible. The feasibility rates considered eligible according to our criteria were evaluated. In addition, we analyzed the reasons for the lack of feasibility were analyzed. The interobserver agreement for the assessment of feasibility before and after training was also calculated.
Results
The overall feasibility rates for both groups was 73%. No significant difference in the feasibility rates was observed. The feasibility rates of each observer for group I were 71% (observer 1), 77% (observer 2) and 70% (observer 3) and those for group II were 73%, 76% and 69%, respectively. In the tumors (n = 164) considered ineligible, the two most common causes for refraining from performing radiofrequency ablation included non-visualization of the tumor (62%) and the absence of a safe route for the percutaneous approach (38%). We found moderate interobserver agreement for all observers before cooperative training and a good agreement after training.
A variety of image-guided tumor ablation techniques have been introduced over the last decade and they play important roles in the therapeutic management of malignant hepatic tumors (1-8). Radiofrequency ablation is one of the local ablation techniques which has received great attention for the treatment of unresectable malignant hepatic tumors (9-13). Ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) can be used for planning, guiding and monitoring local ablation therapies of hepatic tumors. Among these, US has been the most widely used as a guiding modality for percutaneous radiofrequency ablation (14-17) because of its easy availability and real-time monitoring capability (4, 18).
For hepatic tumors to be successfully treated with US guided percutaneous radiofrequency ablation, the tumors are required to be clearly demonstrated on the pretreatment planning US and any critical organs or structures should not be present in the pathway of the radiofrequency electrode. Therefore, the feasibility and treatment strategy should be thoroughly evaluated in performing US for the planning before the procedure (4). We can speculate on the many physicians performing planning US for the candidates of radiofrequency ablation before the procedure. The importance of the operator's experience with radiofrequency ablation has been emphasized in several studies and there is a significant learning curve for the procedure (19). Similarly, the feasibility of performing US guided percutaneous radiofrequency ablation may vary according to the accumulated experience of the operators. Thus, we thought that conducting a cooperative training could reduce the inter-observer variability of the operators and bring the reproducibility to an acceptable level when assessing the feasibility of performing US guided percutaneous radiofrequency ablation for treating hepatic tumors. The improvement in the inter-observer agreement could avoid the unnecessary admission or delayed treatment. To the best of our knowledge, no study has focused on the feasibility of performing US guided percutaneous radiofrequency ablation for treating hepatic tumors.
The purpose of this study was to assess the feasibility of US guided percutaneous radiofrequency ablation for treating hepatocellular carcinoma (HCC) with the performance of pretreatment planning US. As well we also evaluated the inter-observer variability among the operators who had different levels of experience before and after a cooperative training program.
This is a nonrandomized prospective study with approval from the Institutional Review Board and consent from all the patients. The calculated sample size of cases needed in each group was 96 before and after cooperative training with an 80% power value and a 5% α value (the type I error probability for a two sided test), according to the PS Power and Sample Size Calculations (version 2.1.30, Noshville, TN).
Between September 2003 and August 2004, 200 nodular HCCs in 146 consecutive patients were prospectively recruited to undergo planning US before their radiofrequency ablation procedures. We only included the patients who were recommended to undergo US guided percutaneous radiofrequency ablation of HCC after a triage by five hepatologists who had at least 3-years of experience in referring the patients for US-guided radiofrequency ablation. Of the 146 patients, 140 (96%) had risk factors of HCC (liver cirrhosis: 86% and chronic hepatitis: 10%). The levels of α-fetoprotein (AFP) were highly variable with a range of 4-12,794 ng/mL (mean: 15 ng/mL). We divided the patients into two groups: Group I included 74 patients with 100 nodular HCCs which were evaluated between September 2003 and February 2004 before the cooperative training. Group II was composed of 72 patients with 100 HCCs who were evaluated between March 2004 and August 2004 after the cooperative training. The amount of time between the before and after the cooperative training was one month. The clinical characteristics of the patients in each group are summarized in Table 1. All patients had HCCs detected at contrast-enhanced multiphase helical CT. The diagnosis of HCC for 13 tumors was assessed by percutaneous needle biopsy. The remaining 187 tumors were considered to be HCCs on the basis of imaging findings (n = 157, characteristic enhancement patterns at contrast-enhanced multiphasic CT or dynamic contrast-enhanced MR imaging - hypervascularization during the hepatic arterial phase, and a wash-out pattern during the equilibrium phase) and elevated serum tumor marker (n = 5, α-fetoprotein [AFP] level > 400 ng/mL [> 400 µg/L]) or at least two concurrent radiological findings which were compatible with HCC among CT, MR imaging, US, and angiography (n = 25) (20).
All patients met the following criteria for treatment with percutaneous radiofrequency ablation: the presence of a single tumor with a maximum diameter not exceeding 5 cm, or the presence of multiple (not more than four) tumors and each with a maximum diameter not greater exceeding 3 cm; the absence of portal venous thrombosis and extrahepatic metastasis; the presence of liver cirrhosis of Child-Pugh class A or B. Remote recurrent HCCs (n = 88) after hepatectomy (n = 13), percutaneous ethanol injection therapy (n = 2), transarterial chemoembolization (TACE) (n = 13), radiofrequency ablation (n = 39) and combined treatment (n = 21) were also included in the study irrespective of the prior history of treatment. The operability of the patients did not affect the inclusion criteria.
We used 17-gauge internally cooled single or cluster RF electrodes (Radionics, Burlington, MA) with a 500-kHz monopolar generator (series CC-3; Radionics) which was able to produce a 200-W output.
Three abdominal radiologists, who had different levels of experience for the diagnosis of percutaneous radiofrequency ablation of hepatic tumors, independently performed the sonographic examinations for each tumor by themselves. Immediately after one observer finished the sonographic examination for each patient, the others followed within a mean of 5 minutes. They were aware of the CT findings before conducting the sonographic studies. Observer 1 (senior attending staff) assessed more than 1,000 cases of percutaneous radiofrequency ablation under the guidance of US, observer 2 (junior staff) assessed over 150 cases and observer 3 (fellow) had 15 cases of experience without doing the procedure during the research time. All the sonographic examinations were performed using 1.0- to 4.0-MHz convex array probes (Acuson-Sequoia 512, Siemens Medical Solutions, Mountain View, CA) or 2.0- to 5.0-MHz curvilinear probes (HDI 5000; Advanced Technology Laboratories, Philips Medical Systems, Bothell, WA). Using gray-scale and color Doppler US, the observers independently evaluated the feasibility of US guided percutaneous radiofrequency ablation of each tumor. They also archived the sonographic images of each tumor on a picture archiving and communication system (PACS, Centricity Workstation, version 1.0; GE Medical Systems, Milwaukee, WI). The three radiologists scanned 74 patients with 100 nodular HCCs before the cooperative training and they decided the feasibility of the procedure for each patient based on their experience. After the cooperative training, the remaining 72 patients with 100 HCCs were independently examined by the same three radiologists.
After the sonographic examination, they recorded the results for the feasibility of performing US guided percutaneous radiofrequency ablation for each tumor. The probability of completeness of treatment (i.e. incomplete ablation or primary effectiveness) did not affect the feasibility. We simplified the feasibility as "feasible" or "not feasible". In general we considered the following tumors as ineligible for US-guided percutaneous radiofrequency ablation: 1) When the critical structures (large vessels, lung base, gallbladder or gastrointestinal tract) are located in the presumed electrode pathway (i.e. When the gallbladder or gastrointestinal tract interrupts the pathway of electrode, or when we cannot keep away from the segmental portal vein or main portion of hepatic vein or vessel greater than 3 mm in diameter); 2) When the tumor is not visualized on US scans. Whenever the observers considered that percutaneous radiofrequency ablation was not feasible, they were asked to describe the reasons why it was not feasible. This work was done independently by each observer. After the planning US, the final treatment methods of these patients or outcome of radiofrequency ablation were evaluated.
The training sessions were performed after the sonographic studies done for group I (100 nodular HCCs in 74 patients). The modulator (observer 3) arranged the training and prepared all the necessary data only. All three observers participated in the cooperative training for four hours which involved the review of the sonographic images archived on the PACS workstation for all the cases which showed different decisions on the feasibility among the observers. Immediately after reviewing each case, we discussed the reason for the discrepancy and we tried to reach a consensus on the feasibility of performing percutaneous radiofrequency ablation for cases which were not the decision of senior staff. We compared the index tumor on US with the presuming lesion on the CT scan for evaluation of the reason of non-visualized cases. If the tumor had low conspicuity, a radiologist tried to make a higher conspicuity with use of harmonic images and taught it to other radiologists. We evaluated the location of the cases which showed a discrepancy in the feasibility between the operators and analyzed the critical structures which were obstacles to the the safe route to approach the index tumor. We discussed if there were an alternative safe route to approach the tumor. The same process was continued for the all cases with different decisions on feasibility.
The overall feasibility and individual feasibility rates were evaluated before and after the cooperative training. Also, the reasons for all the cases in which percutaneous radiofrequency ablation was considered not feasible were assessed. Moreover, before and after the cooperative training, the inter-observer agreement among the three observers on the feasibility were analyzed. Furthermore, for the cases with a discrepancy between the three observers, the characteristics of the tumors were assessed and the causes of these cases were evaluated. The difference in the numbers showing a discrepancy were assessed before and after cooperative training.
The feasibility rates of the observers between the two groups were statistically analyzed using the Generalized Estimating Equation (GEE) test. To assess the agreement between the observers on the feasibility of performing US guided percutaneous radiofrequency ablation, kappa (κ) values were calculated using a computer program (MedCalc, version 6.0; Medcalc Software, Mariakerke, Belgium). A κ-value greater than zero was considered to be indicative of a positive correlation. The inter-observer agreement was rated as follows: poor, κ<0.20; fair, κ = 0.21-0.40; moderate, κ = 0.41-0.60; good, κ = 0.61-0.80; very good, κ = 0.81-1.00. This was assessed by comparing the Cohen's κ coefficients (21, 22) which were calculated for each pair of observers before and after the cooperative training. Comparisons between the κ values before and after the cooperative training were performed using the Z test.
(K1; κ value of group I, K2; κ value of group II SE1; standard error of group I, SE2; standard error of group II)
A p-value less than 0.05 was considered to indicate a statistically significant difference.
The clinical characteristics of the patients, including the patient profiles and tumor characteristics in the both groups, were not statistically different (Table 1).
The overall feasibility rate judged by the proportion of the tumors considered eligible for US guided percutaneous radiofrequency ablation by all three observers (Figs. 1, 2), was 73% (436/600). The feasibility rates of each observer for group I were 71% (observer 1; 71/100), 77% (observer 2; 77/100) and 70% (observer 3; 70/100) whereas, for group II were 73% (observer 1; 73/100), 76% (observer 2; 76/100) and 69% (observer 3; 69/100), respectively. There was no significant difference in the feasibility rates between the two groups (GEE test, p>0.05).
For all the tumors (n = 164) considered ineligible for US guided percutaneous radiofrequency ablation by all three observers, the most common causes for refraining from performing a radiofrequency ablation procedure included nonvisualization of the tumor (62%, 102/164) and the absence of a safe route for the percutaneous approach (38%, 62/164) (Fig. 3).
The mean tumor size considered ineligible for US guided percutaneous radiofrequency ablation was 1.9 cm (range, 0.7-3.4 cm), whereas the mean tumor size considered eligible was 2.1 cm (range, 0.5-5.0 cm). The difference in size between the two groups was not statistically significant (p > 0.05, student T-test). The reasons for the cases which had no safe treatment route (n = 62) included abuttment on the large hepatic vessels (n = 23), obscured by the lung (n = 19), abuttment on the gallbladder (n = 6), abuttment on the heart (n = 4), exophytic growth (n = 4), abuttment on the diaphragm (n = 2), attachment to the bowel (n = 2), and attachment to the inferior vena cava (n = 2).
After the planning US, 171 patients were treated and among these, 49% (84/171) underwent US guided percutaneous radiofrequency ablation. After radiofrequency ablation, only 5% (4/84) of patients was a residual tumor found and those four patients were treated by TACE for the residual tumor. The rest of the 87 patients were treated with TACE (n = 66), intra-operative radiofrequency ablation (n = 7), surgical resection (n = 9), and liver transplantation (n = 4) (Table 2).
We found moderate (κ-value, 0.41-0.60) inter-observer agreement for all three observers in group I and good inter-observer agreement (κ-value = 0.575) for group II (Table 2). The κ-values between the observers for group I were 0.575 (observers 1 and 2), 0.495 (observers 1 and 3), and 0.431 (observers 2 and 3), whereas those for group II had κ-values of 0.763 (observers 1 and 2), 0.758 (observers 1 and 3), and 0.676 (observers 2 and 3), respectively. The improvement of inter-observer agreement between observers 1 and 3, and 2 and 3 was statistically significant (Z-test) (Table 3) before and after the cooperative training (p<0.05). The different kappa values of observers 1 and 2 were not statistically significant between both groups (p>0.05).
We observed a significant difference in the number of lesions n = 32) with different feasibility assessments between the three observers before the cooperative training and 18 lesions after the cooperative training (p<0.05, Chi-square test). The mean diameter of the coincident cases was 2.2 cm (range, 0.5-5.0 cm) whereas tumors which had different feasibility assessments were on average, 1.8 cm (range, 0.7-3.5 cm) (p < 0.05, Mann-Whitney U test). The frequency of tumor locations was 27% in the left hepatic lobe and 73% in the right hepatic lobe for coincident cases, whereas the frequency was 36% in the left lobe and 64% in the right lobe for tumor with different feasibility assessments. Before the cooperative training, the most common cause of discrepancy was the ability of localization of the index tumor (72%) and the second cause was the different decision to approach the tumor when the critical structures abutted the tumor (28%) (Fig. 4). After cooperative training, the ability of localization of the tumor had improved (44%), however, the improvement was not statistically significant (p>0.05, Chi-square).
To the best of our knowledge, this is the first study which has evaluated the influence of cooperative training on the assessment of the feasibility for the performance of US guided percutaneous radiofrequency ablation for HCC according to operators who have different levels of experience. A learning curve effect regarding the procedure itself has been addressed in another study on the radiofrequency ablation for liver tumors (19).
Radiofrequency ablation has generally been performed with the use of the image guided percutaneous approach (13, 14, 23). US is now accepted as the most popular guiding technique for percutaneous radiofrequency ablation due to its many advantages (4, 14-18). Imaging is used in five separate and distinct ways: planning, targeting, monitoring, controlling and assessing treatment response (4, 24). Imaging aspects are important for planning when measuring the size, shape, number and location of the lesions relative to the critical structures due to the risk of injury during ablation. Accurate targeting during the ablation usually depends on the pretreatment planning, and the accuracy of targeting will determine the treatment response. Therefore, the pretreatment planning US is very important for evaluating the feasibility of US guided percutaneous radiofrequency ablation. Although the percutaneous radiofrequency ablation has been proved safe in many clinical trials, previous reports have not emphasized the importance of operator experience in radiofrequency ablation for hepatic tumors; thus, more attention is occasionally paid to the results and efficacy of the radiofrequency ablation procedure than to the training and experience of the operators. Among the various imaging modalities, the US-guided technique is the most subjective and operator-dependent relative to other modalities such as MRI or CT (25). The results of our study emphasize the importance of cooperative training in order to reduce the inter-observer variability when assessing the feasibility of performing US guided radiofrequency ablation for liver tumors.
By conducting cooperative training among the many operators with different levels of experience, we could attain an acceptable level of reproducibility for all levels of experience. In our institution, many radiologists who have different levels of experience carry out the procedures, from senior staff members with expertise, to fellows with little experience in radiofrequency ablation. When an inexperienced operator performs the procedure, another experienced operator usually supervises them through the entire process of the procedure. There are times when the operators are different from the persons who performed the pretreatment planning US. Consequently, discrepancies in the assessment of the feasibility may happen (Fig. 4). These discrepancies may result in unnecessary admission or delayed treatment. When the patients were not considered good candidates for the US guided percutaneous radiofrequency ablation, we usually recommended other therapeutic modalities including surgery, intraoperative radiofrequency ablation or trans-arterial chemoembolization. These modalities; however, should be scheduled in advance before admission.
In our study, the effect of cooperative training was substantial for observer 3. The change in inter-observer agreement after cooperative training between observers 1 and 2 was not statistically significant, whereas those between observers 1 and 3, and observers 2 and 3 were statistically significant. These results demonstrate that the least experienced operator obtains the greatest benefit from cooperative training. The recent studies (19, 26) suggested that the radiofrequency ablation procedure involves a significant learning curve and cumulative experience of the procedures could decrease the procedure time, complications and increase the success rate of treatment.
The interesting finding in our study was that there was no significant difference in the proportion of the tumors considered feasible for US guided percutaneous radiofrequency ablation among the three operators. This means that approximately a quarter of the tumors referred by the hepatologists are not eligible for the US guided percutaneous radiofrequency ablation, regardless of the operator's level of experience. We believe that this observation is an inherent limitation of US guided radiofrequency ablation for HCC in the patients with cirrhosis. The most common cause of a lack of feasibility for US guided radiofrequency ablation was non-visualization of the tumor with US. It is often difficult to obtain good conspicuity of a tumor with sonographic scanning because of the poor sonic window in the patients with advanced liver cirrhosis, even if the tumor is well delineated at CT. The second common cause for the ineligibility of the condition was the lack of a guaranteed safe route to place the electrode into the tumor, which is essential to the minimization or prevention unintended major complications, including bleeding or injury to the critical structures.
This limitations of this study are the following: First, the patients in the both groups before and after cooperative training were not identical and the differences may have had an influence on the effect of cooperative training. The ideal design is to perform the study on the same patients to avoid the selection bias. However, this would be not possible due to ethical issues: HCC should be treated without delay once it is diagnosed. In this study, there was no statistically significant difference for the clinical characteristics between the two groups, which could have minimized the selection bias. Second, the true learning curve for performing pretreatment planning US for US guided percutaneous radiofrequency ablation was not identified. This issue is not a major concern of the current study and it should be addressed in future studies. Third, since the group I patients were evaluated earlier than the group II, we could not assess the bias of the temporal learning curve, which most greatly affects observer 3 who has the least experienced observer.
In summary, for the pretreatment planning US, 73% of the referred tumors were considered eligible for the US guided percutaneous radiofrequency ablation. Moderate agreement was found among the three operators to assess the feasibility of US guided percutaneous radiofrequency ablation for HCC before the cooperative training. Conducting cooperative training among the operators with different levels of experience was helpful, to a good degree, for improving the inter-observer agreement, which may reduce the unnecessary admission or delayed treatment.
Figures and Tables
Fig. 1
The histogram shows the feasibility rates assessed by the three observers with different levels of experience to perform radiofrequency ablation in liver tumors. No statistical difference in feasibility rates between the two groups before and after cooperative training was observed (GEE test, p>0.05).

Fig. 2
56-year-old man with small hepatocellular carcinoma.
A. Contrast-enhanced transverse CT scan obtained during the hepatic arterial phase shows a 2.0 cm diameter hepatocellular carcinoma (arrow) in liver segment 8.
B-D. Pretreatment planning sonographic scans performed by three different observers show an echogenic mass (arrows) in same corresponding area of liver. All three observers judged that the US guided percutaneous radiofrequency ablation was feasible for this tumor.
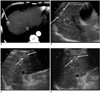
Fig. 3
58-year-old man with two small hepatocellular carcinomas.
A. Contrast-enhanced transverse CT scan obtained during hepatic arterial phase shows a 1.5 cm diameter enhancing tumor (arrowhead) in liver segment 8 and another 1.8 cm in diameter tumor (arrow) in liver segment 7.
B. Pretreatment planning sonographic scans performed by observer 1 shows tumor (arrow) in liver segment 7 with poor conspicuity. The tumor of segment 8 was not visible. In addition to non-visualization of tumors, there was no safe path for the electrode due to the intervening lung base. All three observers considered that US guided percutaneous ablation was not feasible for these tumors.
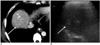
Fig. 4
Discrepancy in assessing feasibility of US guided percutaneous radiofrequency ablation in a 45-year-old man with hepatocellular carcinoma.
A. Contrast-enhanced transverse CT scan obtained during hepatic arterial phase shows a 3.5 cm diameter enhancing tumor (arrow) in liver segment 8.
B-D. On the pretreatment planning sonographic scans performed by observer 1 (B), observer 2 (C), and observer 3 (D), the tumor (arrows) is well delineated as hypoechoic mass. Observer 1 considered that US guided percutaneous radiofrequency ablation was not feasible because of absence of a safe path resulting from the position of the hepatic vein (arrowheads) in B surrounding the tumor. The other two observers thought that the procedure was feasible.
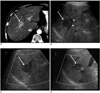
Table 2
Final Treatment Method and Outcome of Sonography Guided Percutaneous Radiofrequency Ablation of the Patients

Table 3
Interobserver Agreement of Three Observers Before and After Cooperative Training

Note-†κ-values: poor, <0.20; fair, 0.21-0.40; moderate, 0.41-0.60; good, 0.61-0.80; very good, 0.81-1.00
‡Z test was used (<0.05)
§Observer 1 (senior attending staff): had more than 1000 cases of experience for percutaneous radiofrequency ablation under the guidance of US
Observer 2 (junior staff): had over 150 cases of experience
Observer 3 (fellow): had 15 cases of experience
References
1. Ahmed M, Goldberg SN. Thermal ablation therapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2002. 13:S231–S244.
2. Dodd GD 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000. 20:9–27.
3. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities-part II. J Vasc Interv Radiol. 2001. 12:1135–1148.
4. Goldberg SN, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003. 228:335–345.
5. Honda N, Guo Q, Uchida H, Ohishi H, Hiasa Y. Percutaneous hot saline injection therapy for hepatic tumors: an alternative to percutaneous ethanol injection therapy. Radiology. 1994. 190:53–57.
6. Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995. 197:101–108.
7. Vogl TJ, Muller PK, Hammerstingl R, Weinhold N, Mack MG, Philipp C, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995. 196:257–265.
8. Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablation of hepatocellular carcinoma: state-of-the-art. Liver Transpl. 2004. 10:S91–S97.
9. Goldberg SN. Comparison of techniques for image-guided ablation of focal liver tumors. Radiology. 2002. 223:304–307.
10. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000. 88:2452–2463.
11. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.
12. Lencioni R, Cioni D, Bartolozzi C. Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging. 2001. 26:345–360.
13. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.
14. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.
15. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.
16. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.
17. Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000. 217:119–126.
18. Gazelle GS, Haaga JR. Guided percutaneous biopsy of intraabdominal lesions. AJR Am J Roentgenol. 1989. 153:929–935.
19. Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004. 239:441–449.
20. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001. 35:421–430.
21. Agresti A. An introduction to categorical data analysis. 1996. New York: Wiley.
22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.
23. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
24. Jolesz FA. Interventional magnetic resonance imaging, computed tomography, and ultrasound. Acad Radiol. 1995. 2:Suppl 2. S124–S125.
25. Choi D, Lim HK, Lee WJ, Kim SH, Kim MJ, Kim SK, et al. Radiofrequency ablation of liver cancer: early evaluation of therapeutic response with contrast-enhanced ultrasonography. Korean J Radiol. 2004. 5:185–198.
26. Danford DA, Kugler JD, Deal B, Case C, Friedman RA, Saul JP, et al. The learning curve for radiofrequency ablation of tachyarrhythmias in pediatric patients. Participating members of the Pediatric Electrophysiology Society. Am J Cardiol. 1995. 75:587–590.
27. Couinaud C. Le Foie: Etudes anatomiques et chirurgicales. 1957. Paris: Masson.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


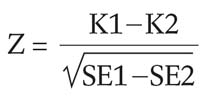

 XML Download
XML Download