Abstract
We present the multidetector CT findings with a pathologic correlation for the case of a solitary fibrous tumor located in the trachea. The MDCT revealed a well-circumscribed intraluminal mass arising from the trachea, with strong nodular enhancement in the periphery of the mass. The enhancement pattern of the mass corresponded histopathologically to a focal hypocellular area in the center and prominent blood vessels along the periphery of the mass. We also present volume-rendered and virtual bronchoscopic images of this rare submucosal tracheal tumor.
Primary benign tumors of the trachea are a rare occurrence, and solitary fibrous tumors (SFTs) that are benign tracheal tumors, are even rarer (1-3). SFTs may occur anywhere in the thoracic or extrathoracic region, but generally occur in the pleura and lungs (4). In only extremely rare cases has a SFT of the trachea been reported in the English language clinical literature (2, 3). To the best of our knowledge, our report is the first to present clinical evidence of CT findings for tracheal SFTs with a pathological correlation.
A 62-year-old woman with dyspnea, and recent aggravation of the condition 10 days prior, was referred to our institution for further evaluation of the tracheal mass. The woman had no outstanding medical history; however, upon a physical examination, a stridor and wheezing sound was examined by auscultation.
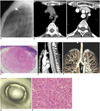
A digital chest radiograph showed a well demarcated mass with round opacity located in the mid level of the trachea (Fig. 1A). A non-contrast enhanced chest CT scan was performed at a local clinic and revealed a well defined, intraluminal mass with homogeneous soft tissue attenuation in the trachea (Fig. 1B). The mass was round in shape with a diameter of 1.5 cm. Next, a contrast-enhanced chest CT (Somatom Sensation 64, Siemens Medical Solutions, Erlangen, Germany) scan revealed a heterogeneous enhancement pattern representing a strong nodular enhancement in the peripheral portion of the mass, accompanied with a central area of low attenuation on the axial images (Fig. 1C). Further, a photomicrograph helped determine that the round submucosal mass was primarily composed of hypercellular areas with some collagen-rich areas. In addition, various sized blood vessels were noted along the periphery of the mass, along with dilated and branched vessels in the central portion of the mass (Fig. 1D).
The oblique sagittal reconstruction images clearly demonstrated the orientation of the mass and length of the tumor (Fig. 1E). In addition, no evidence of tracheal wall invasion was observed. The volume-rendered and virtual bronchoscopic images clearly depict a round intraluminal mass arising from the anterior tracheal wall (Figs. 1F, G). Furthermore, a conventional bronchoscopy revealed the round mass with prominent of small blood vessels on its smooth surface. Although the bronchial mucosa was intact, a bronchoscopic biopsy was not performed with the possibility of massive bleeding. Consequently, the surgical excision of the mass was performed via a segmental resection of the trachea and an end-to-end anastomosis.
A histological examination (Hematoxylin & Eosin staining) revealed a benign SFT with a haphazard growth pattern of short spindle cells with scant cytoplasm and strands of rope-like collagen (Fig. 1H). No evidence of pleomorphism or mitotic activity was observed in the mass. As well, no secondary degeneration was observed within the mass. An immunohistochemical study revealed a positive response for CD34 and negativity for smooth muscle actin, desmin and S100 protein.
Benign tracheal tumors are quite rare, consisting about only 1.9% of all lung tumors. More common benign tumors of the tracheobronchial tree include papillomas, hamartomas, hemagiomas and neurogenic tumors. On the other hand, chondromas, leiomyomas, lipomas and SFTs are a rare occurrence in the tracheobronchial tree (1-3). Furthermore, SFTs are rare spindle cell neoplasms that occur extremely rarely in the trachea. In a study of 185 benign tumors of the tracheobronchial tree, only 2.2% (4 cases) of lesions were identified as SFTs (2, 5).
Much debate exists about the precursor cell of SFTs. A series of names including tracheal fibromas, benign mesothelioma, localized fibrous mesothelioma and submesothelial fibroma have been used to designate these neoplasms. England and colleagues suggested that SFTs originate from a primitive multipotential cell of mesenchymal differentiation (6). SFTs have an inconsistent microscopic appearance and unpredictable biological behavior. Furthermore, SFTs are histologically characterized by a haphazard growth pattern with short spindle cells, scant cytoplasm, a bland cytological appearance and separated by strands of rope-like collagen. Typically, these tumors exhibit a mixture of hypercellular (tumor-rich) and hypocellular (collagen-rich) areas. Most tumors show prominent vascularity, with numerous small and medium-sized blood vessels which focally resemble a hemangiopericytic growth pattern (7). These tumors have a tendency to be positive for CD34 in immunohistochemical studies; however, typically lack the expression for cytokeratin and S-100 protein. In addition, the bcl-2 assay can confirm the diagnosis of SFTs in the case of CD34 negativity (4, 8).
The radiological findings of SFTs occurring in the pleura, head and neck are usually depicted as well-circumscribed soft tissue masses, with lobular or smooth external surfaces (8-10). A non-contrast CT scan of SFTs indicate intermediate to high attenuation (8). Alternatively, contrast-enhanced CT scans demonstrate significant, heterogeneous enhancement. The enhancement pattern perhaps correlates with the vascular nature of these lesions, in addition to secondary degenerations such as myxoid change, hemorrhage, necrosis or cystic degeneration (8, 10).
Similar to the CT findings of SFTs occurring in the pleura, head and neck, the SFTs in this case study have a well-defined round mass with a smooth surface in the trachea. This morphologic feature was compatible with that of the benign tracheal tumor in that it was made up of homogeneous soft tissue attenuation as revealed by a non-contrast CT scan. This finding correlates with the tumor size and histopathology of the mass, which primarily consists of hypercellular (tumor-rich) areas without secondary degeneration. As well, the tumor had multiple small vessels on its surface and peripheral portion of the mass. Moreover, the mass contained less collagen within its periphery. This characteristic resulted in strong nodular enhancement along the periphery of the mass, as seen on the contrast-enhanced CT scan, and is consistent with the CT findings in pleural and extrathoracic SFTs. A contrast-enhanced CT scan also revealed a focal area with a low attenuation in the central portion of the mass, although the present tumor was small in size without secondary degeneration. A previous study reported that the enhancement pattern of SFTs may depend on the amount of collagen within the tumor (10). In addition, another study showed low attenuation in the central area of the pleural SFTs. Correspondingly, a contrast-enhanced CT scan of the tumor contained hypocellular areas of dense fibrosis or loose myxoid stroma, with rich vascularity contributing to the strong enhancement of the mass (11). The heterogeneous enhancement of the present case may have been less conspicuous with delayed CT images, as the mass was primarily composed of hypercellular areas rather than collagen-rich areas.
It is difficult to diagnose a tracheal SFT, since its morphology and enhancement pattern is similar to several benign tracheal tumors including hemagiomas, neurogenic tumors, leiomyomas and glomus tumors (3, 12).
In conclusion, we report, for the first time, the CT and pathologic findings of a SFT of the trachea. Although SFTs of the trachea are extremely rare, SFTs should be included in the suite of the diagnosed benign tracheal masses with a strong enhancement of the CT scan.
Figures and Tables
Fig. 1
62-year-old woman with solitary fibrous tumor of trachea.
A. Lateral chest radiograph revealing well demarcated mass, with round opacity (arrowhead) in mid level of trachea.
B. Non-contrast enhanced CT scan revealing well-defined, round, intraluminal mass with homogeneous soft tissue attenuation in trachea.
C. Contrast enhanced CT scan depicting strong enhancement in peripheral portion of mass with central area of low attenuation.
D. Photomicrograph (Hematoxylin & Eosin staining, × 10) demonstrating round exophytic submucosal mass with various sized blood vessels in its periphery. It is primarily composed of tumor rich areas with some areas of collagen (asterisks).
E. Oblique sagittal image of mass located in mid level of trachea, arising from anterior wall.
F, G. Volume-rendered and virtual bronchoscopic images revealing round intraluminal mass arising from anterior tracheal wall.
H. Histological examination (Hematoxylin & Eosin staining, × 400) revealed haphazard growth pattern of short spindle cells with scant cytoplasm and strands of rope-like collagen. Immunohistochemical study showed positive response for CD34 and negative response for smooth muscle actin, desmin and S100 protein (not shown), which is consistent with solitary fibrous tumor.
References
1. Miller WT Jr. Obstructive diseases of the trachea. Semin Roentgenol. 2001. 36:21–40.
2. Shah H, Garbe L, Nussbaum E, Dumon JF, Chiodera PL, Cavaliere S. Benign tumors of the tracheobronchial tree. Endoscopic characteristics and role of laser resection. Chest. 1995. 107:1744–1751.
3. Ko JM, Jung JI, Park SH, Lee KY, Chung MH, Ahn MI, et al. Benign tumors of the tracheobronchial tree: CT-pathologic correlation. AJR Am J Roentgenol. 2006. 186:1304–1313.
4. Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlation of solitary fibrous tumors. Cancer. 2002. 94:1057–1068.
5. Klemperer P, Rabin CB. Primary neoplasms of the pleura: a report of five cases. Arch Pathol. 1931. 11:385–412.
6. England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura: a clinicopathologic review of 223 cases. Am J Surg Pathol. 1989. 13:640–658.
7. Moran CA, Suster S, Koss MN. The spectrum of histologic growth patterns in benign and malignant fibrous tumors of the pleura. Semin Diagn Pathol. 1992. 9:169–180.
8. Rosade-De-Christenson ML, Abbott GF, McAdams HP, Franks TJ, Galvin JR. From the archives of the AFIP: localized fibrous tumor of the pleura. Radiographics. 2003. 23:759–783.
9. Ganly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, et al. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006. 132:517–525.
10. Levy AD, Rimola J, Mehrotra AK, Sobin LH. Benign fibrous tumors and tumor like lesions of the mesentery: radiologic-pathologic correlation. Radiographics. 2006. 26:245–264.
11. Chong S, Kim TS, Cho EY, Kim J, Kim H. Benign localized fibrous tumour of the pleura: CT features with histopathological correlations. Clin Radiol. 2006. 61:875–882.
12. Koskinen SK, Niemi PT, Ekfors TO, Sipilä J, Valavaara R, Dean PB. Glomus tumor of the trachea. Eur Radiol. 1998. 8:364–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download