Abstract
Percutaneous interventional procedures under image guidance, such as biopsy, ethanol injection therapy, and radiofrequency ablation play important roles in the management of hepatocellular carcinomas. Although uncommon, the procedures may result in tumor implantation along the needle tract, which is a major delayed complication. Implanted tumors usually appear as one or a few, round or oval-shaped, enhancing nodules along the needle tract on CT, from the intraperitoneum through the intercostal or abdominal muscles to the subcutaneous or cutaneous tissues. Radiologists should understand the mechanisms and risk factors of needle tract implantation, minimize this complication, and also pay attention to the presence of implanted tumors along the needle tract during follow-up.
Percutaneous interventional procedures under image guidance play important roles in the management of hepatocellular carcinomas (HCCs). Three common percutaneous interventional procedures are used to manage HCCs: percutaneous biopsy for pathologic diagnosis, percutaneous ethanol injection therapy (PEIT), and percutaneous radiofrequency (RF) ablation. These are usually performed under guidance of ultrasonography, which allows monitoring of the procedures in real-time. Ultrasonography-guidance helps save time relative to other modalities such as computed tomography (CT) or magnetic resonance imaging (MRI).
A variety of immediate or delayed major complications may occur during or after the procedures (1). All of these procedures require insertion of sharp, long needles into the liver parenchyma and tumors, which may cause injury to internal structures and result in hepatic arterial pseudoaneurysm, intrahepatic or subcapsular hematoma, hemoperitoneum, biloma, bile peritonitis, pneumothorax, or infection. The major biopsy complication rates and the associated mortality rates are low, 0.05%-0.18% and 0.006%-0.031%, respectively. In both PEIT and RF ablation, the major complication rates and mortality rates are less than 2% and up to 0.1%, respectively (1-5). However, implantation of tumors along the pathway of the needle can occur during long-term follow-up.
We present the proposed mechanisms, risk factors, frequency, and spectrum of CT findings of needle tract implantation and growth patterns after three common percutaneous interventional procedures, biopsy, PEIT, and RF ablation for HCCs. We also address the lessons learned from our 10-year experience in minimizing tumor implantation.
During percutaneous interventional procedures, viable cancer cells that adhere to the needle can be disseminated along the needle tract during removal. In addition, blood or fluid refluxing back along the tract may carry cancer cells into the peritoneal cavity or to the surface of the skin. Cancer cells may be forced into the tract by a sudden increase in intratumoral pressure, as commonly occurs during RF ablation. Cancer cells have a tendency to spontaneously spread, mainly due to low adhesiveness, low stromal content, and enzyme release following interventional procedures. An experimental study showed that 1,000-10,000 tumor cells could be seeded from a solid tumor along a needle tract (6).
Certain risk factors increase the chances of needle tract implantation, including (a) superficial or subcapsular location of the tumor (7, 8), (b) more needle passes (1, 9), (c) large needle bore (10), (d) the use of an end-cutting needle (1, 7), since specimens obtained by end-cutting needles are often fragmented and lead to greater spillage of tumor cells along the needle tract than a tru-cut needle, (e) the absence or a thin layer of surrounding normal liver parenchyma along the needle tract (1, 9), (f) high-grade (moderate or poor differentiation) HCC (1, 7, 8, 11), (g) high serum -fetoprotein level (8), (h) a tumor larger than 2 cm in diameter enhanced in the early phase on dynamic CT (11), and (i) patient immunosuppression (12).
Despite advanced imaging technologies, HCC imaging features are frequently nonspecific and indeterminate. Percutaneous biopsy is the most important nonsurgical method for histologic diagnosis of focal hepatic lesions, including HCC with different caliber needles (14- to 22- gauge) and different needle passes. The frequency of needle tract implantation of HCC after percutaneous biopsy is between 0-5.1% (7, 9, 10, 13, 14). In our institution, percutaneous biopsy was performed by an abdominal radiologist with the same technique, using either a 19.5-gauge end-cutting (AutoVac®, Angiomed, Karlsruhe, Germany) or an 18-gauge tru-cut (GUNBIOPSY NEEDLE®; Hart, Michigan, MI) needle, with between one and four needle passes. Needle tract implantation developed after percutaneous biopsy in nine (0.76%) of 1,182 patients with HCCs during the recent 10-year period. The implanted tumors were located in the subcutaneous fat and intraperitoneum in three, subcutaneous fat and intercostal muscle in two, intercostal muscle in two, subcutaneous fat and rectus abdominis muscle in one, and subcutaneous fat in one. In eight (89%) of those nine patients, biopsy was performed using an end-cutting needle (range of needle passes, 2 3; mean, 2.8) (Figs. 1-3), and in the other one (11%), the biopsy was performed using a tru-cut needle with four needle passes (Fig. 4). The location of all primary tumors was supracapsular.
Needle tract implantation is possible when the tumor is identified on imaging or physical examination in the perihepatic intraperitoneum, intercostal muscle, abdominal muscle, subcutaneous fat, or cutaneous tissue along the pathway of the needle. CT is the preferred imaging technique for evaluating needle tract implantation and the liver (7, 9). Although implanted tumors may have linear or elongated configurations (Fig. 1), they usually show one or a few, round or oval-shaped enhancing nodules along the previous needle tract (Fig. 2). The tumors can be located from the intraperitoneum through the intercostal or abdominal muscles to the subcutaneous or cutaneous tissues, with or without a linear arrangement (Figs. 1-4) (7, 9, 11). If the lesions grow, they conglomerate and form a relatively large mass (Fig. 3). Although the implanted tumors can reach diameters of 5 cm, they are usually smaller than 2 cm (7, 9, 13). On contrast-enhanced, triple phase CT, the implanted tumors may show hyperattenuation with hypervascularity or isoattenuation relative to the surrounding structures on the arterial phase. Thereafter, they show persistent enhancement with high- or iso-attenuation on portal and equilibrium phases, similar to the typical enhancement pattern (arterial enhancement, followed by washout) of HCC in the liver compared to hepatic parenchymal enhancement (Fig. 4) (7, 9). However, the contrast enhancement pattern may be influenced by the size of the implanted tumors and the characteristics of the primary HCC (9). Different enhancement patterns of implanted tumors and HCCs in the liver may occur due to different blood supplies between the liver and the surrounding tissue where the implanted tumor is located (7).
The growth rate of needle tract implantation after biopsy varies depending on the initial number of inoculated tumor cells and the doubling time of the tumor, as well as the microenvironment surrounding the implanted tumors. The doubling time of the implanted tumors is between 22 and 415 days (mean, 112 days) (7), which is comparable to that of HCC in the liver, or 27 to 605 days (range of means, 102-204 days) (15, 16). The time intervals between biopsy and emergence of needle tract implantation ranged from three weeks to six years (14). In our institution, the time from biopsy to diagnosis of needle tract implantation was four to 21 months (mean, 9 months).
Percutaneous ethanol injection therapy is a low risk, well-established treatment for small HCCs, but can lead to needle tract implantation (11, 12, 17). Although alcohol was thought to sterilize the needle tract and prevent implantation after PEIT (12), the frequency of needle tract implantation is between 0.2%-1.4% (11, 17). PEIT may increase the risk of needle tract implantation compared with biopsy alone because PEIT requires multiple needle punctures over an extended period, despite the advantage of a smaller caliber needle (21-22 gauge) (11). In our institution, PEIT was performed by one of four experienced abdominal radiologists with the same technique. The needles used for PEIT were 21-gauge needles with three side holes and no end hole (PEIT Needle; Hakko, Tokyo). Tumor diameter dictated the amount of alcohol injected: 8 ml for tumors 1 cm in diameter, 15 ml for tumors 2 cm in diameter, and 25 ml for tumors 3 cm in diameter. Injections were performed two or three times a week, depending on patient tolerance, until the total amount of alcohol injected reached the intended volume. Three to eight milliliters of ethanol were administered to each tumor until the ethanol distributed throughout a tumor or until ethanol leakage from a tumor was observed. There was one case (1.9%) of needle tract implantation in the subcutaneous fat among 53 patients who underwent conventional, multisession PEIT (mean session per tumor, 3.7) during the recent 10-year period (Fig. 5). Multiple needle punctures might cause needle tract implantation, as would failing to perform preventive ablation of the needle tract, although the tumor was centrally located. There are no reports of growth rate for these tumors, but Ishii et al. (11) reported that the time intervals between PEIT and diagnosis of needle tract implantation ranged from 10 to 46 months (mean, 21 months).
Radiofrequency ablation is a safe and effective treatment for malignant tumors, especially HCCs. The goal of RF ablation is to kill the index tumor as well as an ablative margin, a peripheral margin of 0.5 to 1.0 cm of normal hepatic parenchyma surrounding the tumor. Each ablation requires exact placement of the electrode tip in the tumor and, when necessary, multiple overlapping ablations are performed according to the morphology of the tumor (18). RF ablation uses 14- to 17-gauge needles, and the frequency of needle tract implantation after RF ablation ranges from 0% to 4.4% (19, 20), although a rate of 12.5% has been reported by one center (8). In our institution, RF ablation was performed by one of four experienced abdominal radiologists with the same technique. From April 1999 to June 2000, 15-gauge multitined expandable electrodes (model 500 series and 1,500 series, Radiofrequency Interstitial Thermal Ablation Medical System, Mountain View, CA or RF 2000 system, RadioTherapeutics Corporation, Mountain View, CA) were used exclusively. In July 2000, we began to use 17-gauge internally cooled electrodes (Cool-tip, Valleylab, Boulder, CO). All patients were treated under intravenous conscious sedation and local anesthesia. We usually prefer the internally cooled electrode for the treatment of tumors near the large, central vessels close to the hepatic hilum and near the gallbladder, colon, and stomach that could be damaged by the fully deployed tines of the multitined expandable electrode. Whenever possible, multiple overlapping ablations (2-6 overlapping ablations; mean, 2.6) were performed for tumors larger than 2.5 cm in diameter. During the recent 10-year period, five cases (1.2%) of needle tract implantation were detected among 414 patients who underwent percutaneous RF ablation without percutaneous biopsy (Fig. 6). Of these, four patients were treated with a multitined expandable electrode and one patient was treated with an internally cooled electrode. The primary tumors were all subcapsular, and the implanted tumors were found in the intraperitoneum in two, intraperitoneum and intercostal muscle in two, and subcutaneous fat in one.
The ablation of the needle tract after RF ablation, use of an internally cooled electrode, and smaller treatment sessions could reduce needle tract implantation compared with PEIT. One study (8) reported a higher incidence (12.5%) of needle tract implantation using an internally cooled electrode, with subcapsular primary tumors and no ablation of the needle tract. However, no reports address whether multitined expandable electrodes or internally cooled electrodes have a higher incidence of needle tract implantation (8). We routinely perform needle tract ablation and use an internally cooled electrode, which may reduce the incidence of needle tract implantation, and could explain our lower incidence than seen in that report (8). The imaging findings of needle tract implantation after percutaneous RF ablation were similar to percutaneous biopsy and PEIT (Fig. 6), as well as previous reports (8, 19).
Needle tract implantation after percutaneous RF ablation would presumably have the same growth profile as percutaneous biopsy and PEIT. Llovet et al. (8) reported that the time intervals between RF ablation and diagnosis of needle tract implantation ranged from four to 18 months (mean, 8.8 months). In our institution, the time interval between RF ablation and diagnosis of needle tract implantation ranged from four to 13 months (mean, 8.8 months).
Based on our 10-year experience, percutaneous needle biopsy should be performed only when necessary, as for determining if a lesion is benign or if pathologic confirmation is necessary prior to nonsurgical therapies, including transcatheter arterial chemoembolization, PEIT, RF ablation, or chemotherapy.
The most important principles for avoiding needle tract implantation are to minimize the number of punctures, the frequency of repositioning the needles, and to traverse a sufficient portion of normal hepatic parenchyma, particularly in subcapsular tumors. In percutaneous biopsy, reducing piston-like movement of the needle, absorbing debris using negative pressure, or using a tru-cut needle can diminish the fragmentation of malignant tissue and minimize spillage of tumor cells along the needle tract. A single pass using a larger bore needle may be better than multiple passes using smaller bore needles (21).
Using larger volumes of alcohol during each needle placement for PEIT may decrease the risk of needle tract implantation because of the sterilizing effect of alcohol along the needle tract and fewer punctures (12). Ablation of the needle tract may alo help minimize needle tract implantation (19). When using the internally cooled electrode for RF ablation, sufficient RF energy should be maintained for minimizing needle tract implantation by viable cancer cells adhered to the electrode after cooling.
However, we learned that needle tract implantation still occurs with some frequency, necessitating careful follow-up of patients, particularly when tumor markers increase in spite of well-controlled hepatic disease.
Although needle tract implantation does not usually affect patient survival (7, 9, 11-14, 17), it may change a potentially curable HCC into an untreatable situation via tumor dissemination to other organs or the peritoneum (22). Therefore, minimizing needle tract implantation is important, as is detection early during tumor growth, which can then be treated successfully by surgical excision (7, 9). Twelve of 15 patients with localized needle tract implantation in our institution were treated with surgical excision. Implanted tumors were not treated in the remaining three patients because of recurrent HCCs in the liver and/or a poor medical condition. Needle tract implantation can alo be managed by ablation, embolization, or radiation therapy (10-12, 14, 17).
Percutaneous interventional procedures such as biopsy, PEIT, and RF ablation are useful in managing HCCs. Although uncommon, needle tract implantation occurs with an incidence that is not negligible, ranging from 0.76% to 1.9% in our institution. All implantations show similar imaging findings, appearing as one or a few, round or oval-shaped enhancing nodules along the previous needle tract on CT, from the intraperitoneum through the intercostal or abdominal muscles to the subcutaneous or cutaneous tissues. During intervention procedures, radiologists should minimize the number of punctures and the frequency of needle repositioning, as well as traverse a sufficient portion of normal hepatic parenchyma, especially for subcapsular tumors, to minimize needle tract implantation. The use of a tru-cut needle for biopsy, larger volumes of alcohol during needle placement for PEIT, and an ablation of the needle tract during RF ablation may help minimize needle tract implantation. During follow-up, radiologists should pay attention to the presence of implanted tumors along the needle tract for early diagnosis.
Figures and Tables
Fig. 1
65-year-old woman with linear or elongated configuration of needle tract implantation after percutaneous biopsy of subcapsular hepatocellular carcinoma.
A. Contrast-enhanced CT image showing 7-cm-diameter, subcapsular hepatocellular carcinoma (arrows). This patient underwent percutaneous biopsy using end-cutting needle.
B. Contrast-enhanced CT image showing linear soft tissue attenuation lesion (arrow) seen in subcutaneous fat layer of right anterolateral chest wall four months after percutaneous biopsy. Note lipiodol-laden primary tumor after transcatheter arterial chemoembolization (arrowheads).
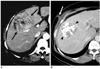
Fig. 2
50-year-old man with needle tract implantation with round or oval-shaped configuration and linear arrangement after percutaneous hepatocellular carcinoma biopsy.
A. Contrast-enhanced CT image showing small, round nodule (arrow) in peritoneal cavity along previous needle tract three months after percutaneous biopsy using end-cutting needle. Note lipiodol-laden primary tumor (arrowhead) after transcatheter arterial chemoembolization.
B. Follow-up CT image showing two round nodules with rapid growth (arrows) in peritoneal cavity and subcutaneous fat layer six months after percutaneous biopsy.
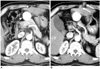
Fig. 3
66-year-old woman with needle tract implantation with large conglomeration around rib after percutaneous hepatocellular carcinoma biopsy, followed by right hepatic lobectomy.
A. Contrast-enhanced CT image showing isoattenuation nodules (arrows) relative to intercostal muscles in intercostal muscles and subcutaneous fat layer five months after percutaneous biopsy using end-cutting needle.
B. Follow-up CT image showing conglomerated nodules (arrows) with increased size nine months after percutaneous biopsy.
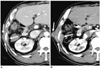
Fig. 4
63-year-old man with needle tract implantation after percutaneous biopsy using tru-cut needle.
A. Contrast-enhanced CT image obtained on arterial phase showing well-demarcated, hyperattenuating hypervascular nodule (arrow) relative to intercostal muscles in right chest wall.
B, C. Contrast-enhanced CT images obtained on portal (B) and equilibrium (C) phases showing nodule (arrows) with persistent enhancement and hyperattenuation relative to intercostal muscles in right chest wall. Note subcapsular lipiodol-laden mass (arrowhead in B).
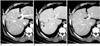
Fig. 5
89-year-old man with needle tract implantation after five times of percutaneous ethanol injection therapy for centrally-located hepatocellular carcinoma.
A. Contrast-enhanced CT image obtained three months after last percutaneous ethanol injection therapy in right liver shows small tumor (arrow) in subcutaneous fat layer.
B, C. Follow-up CT images obtained on arterial (B) and equilibrium (C) phases 51 months after percutaneous ethanol injection therapy showing size increase of implanted tumor (arrows) in subcutaneous fat layer. Imaging findings were similar to needle tract implantation after percutaneous biopsy.
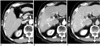
Fig. 6
72-year-old man with needle tract implantation after percutaneous radiofrequency ablation of hepatocellular carcinoma, with history of transcatheter arterial chemoembolization.
A-C. Contrast-enhanced CT images obtained on arterial (A), portal (B), and equilibrium (C) phases 13 months after percutaneous radiofrequency ablation using multitined expandable electrode for viable tumor within partial lipiodol-laden mass. Images show two small enhancing lesions (arrows) involving intercostal muscles along previous needle tract. Imaging findings were similar to needle tract implantation after biopsy and percutaneous ethanol injection therapy. Note subcapsular lipiodol-laden mass (arrowhead) with no residual viable tumor after percutaneous radiofrequency ablation.
References
1. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Radiology. 1991. 178:253–258.
2. Livraghi T, Damascelli B, Lombardi C, Spagnoli I. Risk in fine-needle abdominal biopsy. J Clin Ultrasound. 1983. 11:77–81.
3. Smith EH. The hazards of fine-needle aspiration biopsy. Ultrasound Med Biol. 1984. 10:629–634.
4. Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long term results of percutaneous ethanol injection. Radiology. 1995. 197:101–108.
5. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–136.
6. Ryd W, Hagmar B, Eriksson O. Local tumour cell seeding by fine-needle aspiration biopsy. A semiquantitative study. Acta Pathol Microbiol Immunol Scand. 1983. 91:17–21.
7. Chang S, Kim SH, Lim HK, Lee WJ, Choi D, Lim JH. Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: evaluation doubling time, frequency, and features on CT. AJR Am J Roentgenol. 2005. 185:400–405.
8. Llovet JM, Vilana R, Bru C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001. 33:1124–1129.
9. Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000. 25:246–250.
10. Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma - a study based on 420 patients. J Hepatol. 1996. 25:334–338.
11. Ishii H, Okada S, Okusaka T, Yoshimori M, Nakasuka H, Shimada K, et al. Needle tract implantation of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1998. 82:1638–1642.
12. Arrive L, Vurgait A, Monnier-Cholley L, Lewin M, Balladur P, Poupon R, et al. Long-term follow-up after neoplastic seeding complicating percutaneous ethanol injection for treatment of hepatocellular carcinoma. Eur Radiol. 2002. 12:74–76.
13. Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000. 6:67–72.
14. Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006. 187:1184–1187.
15. Okazaki N, Yoshino M, Yoshida T, Suzuki M, Moriyama N, Takayasu K, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time: a preliminary report. Cancer. 1989. 63:2207–2210.
16. Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992. 16:132–137.
17. Tarantino L, Francica G, Esposito F, Pisaniello D, Parmeggiani D, Marzullo G, et al. Seeding from hepatocellular carcinoma after percutaneous ablation: color Dopper ultrasound findings. Abdom Imaging. 2006. 31:69–77.
18. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
19. Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005. 16:485–491.
20. Bolondi L, Gaiani S, Celli N, Piscaglia F. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001. 34:608.
21. Schotman SN, De Man RA, Stoker J, Zondervan PE, Ijzermans JN. Subcutaneous seeding of hepatocellular carcinoma after percutaneous needle biopsy. Gut. 1999. 45:626–627.
22. Liu YW, Chen CL, Chen YS, Wang CC, Wang SH, Lin CC. Needle tract implantation of hepatocellular carcinoma after fine needle biopsy. Dig Dis Sci. 2007. 52:228–231.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download