Abstract
Objective
To access the feasibility of clinically available 3T MRI to detect the migration of labeled neural stem cells (NSCs) in intracerebral hemorrhage (ICH) in a rat model.
Materials and Methods
The ethics committee of our institution approved this study. ICH was induced by the injection of collagenase type IV into the right striatum of ten Sprague-Dawley rats. Human NSCs conjugated with Feridex (super-paramagnetic iron oxide: SPIO) were transplanted into the left striatum one week after ICH induction. MRI was performed on a 3T scanner during the first, second, third, fourth, and sixth weeks post-transplantation. MRI was obtained using coronal T2- and T2*-weighted sequences. Two rats were sacrificed every week after in vivo MRI in order to analyze the histological findings.
Results
ICH in the right striatum was detected by MRI one and two weeks after transplantation without migration of the NSCs. There was no migration of the NSCs as seen on the histological findings one week after transplantation. The histological findings two weeks after transplantation showed a small number of NSCs along the corpus callosum. On MRI three weeks after transplantation, there was a hypointense line along the corpus callosum and decreased signal intensity in the right periventricular region. Histological findings three weeks after transplantation confirmed the presence of the hypointense line representing SPIO-labeled NSCs. MRI four and six weeks after transplantation showed a hypointense spot in the right periventricular region. The histological findings four and six weeks after transplantation showed the presence of prominent NSCs in the right periventricular region.
Cell therapy using neural stem cells (NSCs) can relieve dysfunctional neurons with certain growth factors and give rise to neurons, astrocytes, and oligodendrocytes to replace the injured cells in tumorous conditions, stroke, and neurodegenerative disease (1-4). Intracerebral hemorrhage (ICH) represents at least 10% of all strokes in Western populations (5) and a considerably higher proportion of strokes in the Asian populations (6). Jeong et al. (7) reported that intravenously transplanted NSCs could enter the rat brain with ICH, undergo migration, and thus improve functional recovery.
Most techniques for the evaluation of stem cell therapy in animal models require histological analysis to determine the fate and migration of cells. Therefore, the number and location of NSCs transplanted into the central nervous system can only be estimated postmortem, thus permitting observation at only one point in time. A technique for continuous and non-invasive detection of the expansion, migration and fate of the transplanted cells is crucial to develop in order to guide further advances in neurotransplantation research and future clinical applications.
Recently, the migration of implanted NSCs can be detected serially with the use of high magnetic field experimental MR scanners such as 4.7T, 7T or 9.4T (8-11). Experimental MR scanners provide high-resolution images and thin slice thickness sufficient to detect a small number of labeled cells. However, the major drawbacks are a long scanning time and heat generation. Currently, high magnetic field MR scanners cannot be used in clinical practice. Therefore, to assure the localization of NSCs and to evaluate the therapeutic effect of NSCs in human trials, labeled NSCs must be detected and monitored on clinically available MRI. Therefore, the purpose of this study is to assess the feasibility of clinical 3T MRI equipment to detect labeled NSCs in ICH in a rat model.
Immortalized human NSCs using a retroviral vector carrying v-myc and vascular endothelial growth factor (HB1.F3.VEGF) were maintained at a density of 1 × 106 cells/100-mm dish (Sarstedt, Sparks, NV) in Dulbecco's modified Eagle's medium with high glucose (DMEM, Hyclone, Logan, UT) containing 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin-streptomycin (Hyclone). Cells were grown to 80% confluence in a 37℃ humidified incubator with 5% CO2 and 95% air environment, and were then harvested in trypsin containing EDTA (Hyclone). The cells were cultured in 100-mm dishes (Sarstedt) coated with poly-L-lysine (Sigma, St Louis, MO).
The Institutional Committee on Animal Research approved this study. Ten Spraque-Dawley rats (250-300 g; Samtako, Osan, Korea) were used in this study. ICH was induced by stereotaxic, intrastriatal administration of collagenase. After an intraperitoneal injection of 1% ketamine (30 mg/kg) and xylazine hydrochloride (4 mg/kg), the rats were placed in a stereotaxic frame (Lab Standard™, Stoelting, Wood Dale, IL). A burr hole was made, and a 26-gauge needle was inserted through the burr hole into the striatum (AP: +1.0 mm, ML: -2.6 mm, DV: -5.0 mm). ICH was induced by the administration of collagenase type IV (1 µl saline containing 0.23 U, Sigma) over a period of five minutes. The needle was then gently removed, the burr hole was sealed with bone wax, and the wound was sutured. Animals were maintained in separate cages at room temperature (25℃), with free access to food and water under a 12-hour light-dark cycle.
Super-paramagnetic iron oxide particles (SPIO, Feridex®, Berlex Imaging, Wayne, IN) were used to label the NSCs (HB1.F3.VEGF). Protamine sulfate (PS, Sigma) was used as a transfection agent (TA). Five µg/µl SPIO and 0.25 µg/µl PS were added to the culture medium, which was incubated at room temperature for 10 minutes with occasional stirring. The growth medium for HB1.F3.VEGF cells was replaced with this solution and was incubated for 48 h. Labeled cells were harvested for transplantation by gentle trypsinization. To remove excess iron oxide particles, trypsinized cells were washed in phosphate buffered saline and the cells were concentrated by centrifugation, resuspended in phosphate buffered saline, and kept on ice.
For transplantation of labeled NSCs, rats were stereotaxically fixed under anesthesia with intraperitoneal injection of 1% ketamine (30 mg/kg) and xylazine hydrochloride (4 mg/kg) a week after ICH induction. Two microliters of 1 × 106 labeled NSCs were delivered to the transplantation site (left striatum) through a Hamilton syringe using a microinjector (Microinjector model 310 plus, KD Scientific, Holliston, MA) at the speed of 0.5 µl/min. Needles were left in the transplantation site for several minutes in order to minimize cell damage caused by negative pressure.
To determine the sensitivity of 3T MRI for the detection of labeled NSCs, duplicate aliquots of decreasing numbers of SPIO-labeled NSCs (2 × 105, 5 × 104, 5 × 103, 250) were injected into a 2% gelatin tube using a microinjector with a Hamilton syringe. Centrifugation was performed to remove microbubbles from the gelatin tube. Each of the gelatin tubes were then placed in a custom-made holder for the experiments performed using a 3T MR scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany) and clinically available wrist coil. The imaging parameters were as follows: TR/TE = 231/10 msec, flip angle = 25°, field of view (FOV) = 7.0 cm, matrix number = 256 × 192, slice thickness = 2 mm and number of excitation = 2.0.
Rats with grafted SPIO-labeled NSCs were examined for period of 1-6 weeks (1, 2, 3, 4, and 6 weeks) post-transplantation using a 3T MR scanner and clinically available wrist coil. Rats were anesthetized for imaging using general inhalation anesthesia (1.5% isoflurane in a 1:2 mixture of O2/N2) during the MRI procedures. To reduce artifacts during respiration, each rat head was fixed in the prone position. T2-weighted images (TR/TE = 3,000/100 msec, flip angle = 150°, acquisition time = 2' 42") and T2*-weighted images (TR/TE = 231/10 msec, flip angle = 25°, acquisition time = 2' 56") were obtained. Other imaging parameters were as follows: FOV = 7.0 cm, matrix number = 256 × 192, slice thickness = 2 mm, slice gap = 0.1 mm and number of excitation = 2.0.
Image analysis was performed before the pathological results were known. Two radiologists in consensus analyzed all images. MR images were reviewed on a picture archiving and communication systems (PACS; Marotech, Seoul, Korea) workstation. T2-weighted images were used for detection of ICH and T2*-weighted images were used to track the labeled NSCs. The labeled NSCs were supposed to present as hypointense foci in brain parenchyma because of a magnetic susceptibility artifact. One radiologist performed quantitative analysis for all of the MR images. The change of signal intensity (SI) ratio on a T2*-weighted image was measured in defined regions of interest in both periventricular regions.
After in vivo MRI, two rats were sacrificed by intravenous injection of pentobarbitone sodium each week. The brains were removed and fixed for 24 h in 4% paraformaldehyde. A coronal section was made through the needle entry site and was then fixed in paraffin. Sections of 7 µm thickness were cut. Hematoxylin and eosin (H & E) stain, Prussian blue stain, and an immunohistochemical stain using an anti-nestin human specific monoclonal antibody (Chemicon, Temecula, CA) were used for a histological examination by one pathologist. Prussian blue stain was used to detect and track the labeled NSCs. Immunohistochemical staining was used to confirm the presence of NSCs.
Prussian blue stain was used for the evaluation of labeling of NSCs. NSCs were efficiently labeled and SPIO located inside cells was visualized by Prussian blue staining. The iron oxide nanoparticles in colonies of NSCs in culture were observed as blue spots on microscopy (Fig. 1).
To check the sensitivity of the MRI technique and to mimic the signal behavior in brain tissue, we performed in vitro MRI of labeled cells. NSCs were labeled with SPIO as described above, and a cell suspension (at concentration of 2 × 105, 5 × 104, 5 × 103, 250 cells/µl) was suspended in 2% gelatin. MR images showed a clear hypointense signal at all concentrations greater than 5 × 103 cells (Fig. 2).
One week after implantation of NSCs, T2-weighted imaging showed ICH in the right striatum. Hypointensity on MRI was found only at the injection site of labeled NSCs and migration of labeled NSCs was not detected. Migration of NSCs into the corpus callosum or the right periventricular region was not seen on the histopathological findings (Fig. 3).
On MRI two weeks after implantation, the ICH finding in the right striatum had not changed. Migration of NSCs did not occur except a hypointense spot at the injection site of the NSCs, decreased slightly in size as seen on T2*-weighted imaging. Prussian blue staining showed fewer blue spots along the corpus callosum, and immunohistochemical staining confirmed migrating NSCs (Fig. 4).
On MRI three weeks after implantation, T2-weighted imaging showed a further decrease in the size of ICH in the right striatum. T2*-weighted imaging showed a decreased size of the hypointense spot in the left striatum and linear hypointensity along the corpus callosum, which is considered to represent labeled NSCs. There was also a small, hypointense spot in the right periventricular region. Prussian blue staining and immunohistochemical staining showed a large number of labeled NSCs along the corpus callosum and right periventricular region (Fig. 5).
MRI after four and six weeks showed similar findings of decreased size of ICH in the right striatum on T2-weighted imaging and an increased hypointense spot in the right periventricular region on T2*-weighted imaging. On T2*-weighted imaging, the hypointense spot in the left striatum had further decreased in size. Prussian blue staining and immunohistochemical staining showed labeled NSCs in the right periventricular region (Fig. 6).
Changes of the SI ratio in the right periventricular region showed a rapid drop between two and three weeks, which was well correlated with the histological findings of large-scale migration of labeled NSCs. On the contrary, the SI ratio at the implantation site of the left striatum gradually increased (Fig. 7).
In our study, clinically available 3T MRI was acceptable to use for the detection of implanted NSCs crossing the corpus callosum to the periventricular region, and this finding correlated with the histopathologic findings. Migration through the corpus callosum to the lesion site in a stroke model has been reported in several studies (11, 14-15). Almost all of the reported studies used experimental MRI (> 4.7T) that enabled in vivo imaging with near microscopic resolution (9-11, 16, 17). Detection of NSCs using experimental MRI has also been achieved using extremely long measurement times of several hours to achieve the necessary signal-to-noise ratio and contrast (9, 16).
Contrast agents for monitoring cell migration on MRI can be divided into two types. One type is an agent containing gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA), and the other type is an agent containing SPIO. As stem cells labeled with Gd-DTPA can be monitored for up to seven days after injection, it is not appropriate for long-term monitoring. Stem cells labeled with SPIO are appropriate for long-term, in vivo monitoring because of the stability and high contrast of SPIO (14, 18). We used SPIO (Feridex®) in our study as it is a contrast agent based on dextran-coated SPIO nanoparticles and the FDA clinically approves it as a blood pool agent.
Particles of SPIO can be as small as 7-10 nm and are located in unmagnetized cell cytosol. SPIO can be detected with high magnetic MRI T2*- as well as on T2-weighted imaging because of its susceptibility artifact (14). SPIO nanoparticles have been used for labeling cells for in vivo MR cell tracking (15). Frank et al. (14) showed that magnetic labeling of mammalian cells using ferumoxides and TAs is possible and may enable cellular MRI and tracking in both experimental and clinical settings. However, anionic surface charges of ferumoxides and TAs transverse negatively charged cell membranes. It is mandatory to use a TA to promote intracellular incorporation of SPIO. Labeling a cell with a contrast agent based on SPIO nanoparticles before transplantation has been widely investigated for use in human medicine (19-20). Widely used TAs are poly-L-Lysine (PLL) and protamine sulfate. PLL is not approved by the FDA and is cytotoxic at a concentration of 10 µM (21-22).
The FDA has approved the use of protamine sulfate as an antidote of heparin, and it is safe and effective as a TA (22). In our study, we could label NSCs effectively with SPIO and protamine sulfate.
SPIO is detectable with at least 500 cells in a plane by the use of 4.7T MRI and 100 cells in a plane by the use of 9.4T MRI (10). High magnetic field MRI (> 4.7T) has a higher sensitivity than 3T MRI for the detection of SPIO due to the high susceptibility effect of high magnetic field MRI. In our study, 5 × 103 cells with a 2-mm slice thickness could be detected on 3T MRI. It is difficult to compare the results of various reports because the sensitivity of labeled stem cells varies according to the magnetic strength, surface coil, and various parameters.
Various routes have been tested for the administration of NSCs (8-10, 12, 23, 24). A greater number of NSCs were recruited around the lesion site when ipsilateral intraparenchymal or intraventricular injection was used rather than contralateral intraparenchymal injection (2). There was no difference in the recruited cell numbers between ipsilateral intraparenchymal injection and intraventricular injection. This finding was thought to be due to the greater probability of survival of the normal parenchyma than of the injured parenchyma. However, intravenous injection required the use of more stem cells and increased the risk of occlusive venous disease caused by thrombus formation and an obstructive lesion or mass formation in the spleen, kidney, or lung (25, 26). We used contralateral intraparenchymal injection of NSCs to increase survival of stem cells and to monitor the intraparenchymal route of the implanted NSCs (2). The optimal method of implantation should be further investigated in order to be able to deliver stem cells safely and effectively in the future.
In our study, MR findings three weeks after implantation showed hypointensity along the corpus callosum, and a drop in the SI in the periventricular region, both of which suggest that migration of NSCs is prominent from two weeks to three weeks after implantation. A histological examination showed inhomogenous distribution of the NSCs at the lesion site, especially in the periventricular region; this correlated well with the MR findings. Our results agree with the findings of Hoehn et al. (9), who reported that SPIO-labeled embryonic stem cells injected intracerebrally could migrate into an ischemic lesion induced by occlusion of the middle cerebral artery. However, in our study, 3T MRI could not detect migration along the corpus callosum at two weeks after implantation despite the presence of a small number of NSCs in the corpus callosum, as detected on the histological findings. This difference can be explained by the lower spatial resolution of 3T MRI than experimental high magnetic field MRI. On 3T MRI, acceptable images could be obtained with a 2-mm slice thickness as the signal-to-noise ratio is decreased with a slice thickness less than 2-mm. Therefore, small numbers of NSCs may not be detectable on 3T MRI.
In conclusion, we have demonstrated that clinically available 3T MRI can detect the migration of NSCs along the corpus callosum in rats with ICH. NSCs were effectively labeled with SPIO using protamine sulfate as a TA. NSCs labeled with iron oxide nanoparticles, remained viable and were able to migrate into an ICH site. Therefore, in the future, 3T MRI may be useful to monitor the migration of NSCs in the clinical setting of stem cell therapy.
Figures and Tables
Fig. 1
Prussian blue staining of neural stem cells (objective magnification: × 40).
A. Unlabeled neural stem cells without blue spots.
B. SPIO-labeled neural stem cells show blue spots located inside cells, suggesting presence of iron oxide particles.

Fig. 2
In vitro T2*-weighted images of neural stem cells.
A-D. 250 neural stem cells in 2% gelatin tube (A), 5 × 103 neural stem cells (B), 5 × 104 neural stem cells (C), 2 × 105 neural stem cells (D). In B, C, and D, T2*-weighted images (TR/TE = 231/10 msec) show linear, hypointense cluster in bottom of tubes (arrows), representing SPIO-labeled neural stem cells.
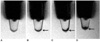
Fig. 3
MR and histological findings one week after implantation of neural stem cells.
A. T2-weighted image (TR/TE = 3,000/100 msec) shows hypointense, SPIO-labeled, neural stem cells (curved arrow) in left striatum and hyperintense lesion (arrows) in right striatum, representing intracerebral hemorrhage.
B. T2*-weighted image (TR/TE = 231/10 msec) reveals hypointense, SPIO-labeled neural stem cells (curved arrow) in left cerebral hemisphere.
C. Hematoxylin and eosin staining (objective magnification: × 4) shows intracerebral hemorrhage (arrows) in right striatum with mass effect to corpus callosum and right lateral ventricle (LV).
D. Prussian blue staining (objective magnification: × 4) shows no migration of SPIO-labeled neural stem cells.
E. Immunohistochemical staining (objective magnification: × 4) shows no migration of neural stem cells.
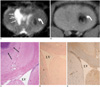
Fig. 4
MR and histological findings two weeks after implantation of neural stem cells.
A. On T2-weighted image (TR/TE = 3,000/100 msec), size of intracerebral hemorrhage (arrow) in right cerebral hemisphere is smaller than it was one week after implantation of neural stem cells.
B. T2*-weighted image (TR/TE = 231/10 msec) shows slightly decreased size of hypointense spot (curved arrow) in left cerebral hemisphere. There is no migration of neural stem cells.
C. Prussian blue staining (objective magnification: × 4) shows small number of neural stem cells in corpus callosum (arrows).
D. Immunohistochemical staining (objective magnification: × 4) shows migration of small number of neural stem cells into corpus callosum (arrows).
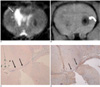
Fig. 5
MR and histological findings three weeks after implantation of neural stem cells.
A. T2-weighted image (TR/TE = 3,000/100 msec) shows decreased size (arrow) of intracerebral hemorrhage two weeks after implantation of neural stem cells.
B. T2*-weighted image (TR/TE = 231/10 msec) shows decreased size of hypointense spot (curved arrow) in left striatum. Linear low signal intensity (arrows) along corpus callosum is considered to represent SPIO-labeled neural stem cells. There is small, hypointense spot in right, periventricular region (arrowheads), suggestive of migrated neural stem cells.
C. Prussian blue staining (objective magnification: × 2) shows large number of SPIO-labeled, neural stem cells in corpus callosum (arrows).
D. Immunohistochemical staining (objective magnification: × 2) shows neural stem cells in corpus callosum (arrows).
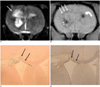
Fig. 6
MR and histological findings four weeks after implantation of neural stem cells.
A. T2-weighted image (TR/TE = 3,000/100 msec) shows decreased size of intracerebral hemorrhage three weeks after implantation of neural stem cells.
B. T2*-weighted image (TR/TE = 231/10 msec) shows decreased size of hypointense spot (curved arrow) in left striatum. Clusters of hypointensity (arrows) are seen in right periventricular region.
C. Prussian blue staining (objective magnification: × 4) shows inhomogeneous distribution of neural stem cells around right ventricle (arrows).
D, E. Immunohistochemical staining (objective magnification: × 4 and × 10) shows neural stem cells in right, periventricular region (arrows).
Fig. 7
Signal intensity ratio changes in MR images of right periventricular region and of left striatum.
Significant decrease of signal intensity ratio between two weeks and three weeks after implantation of neural stem cells is seen in right periventricular region, suggesting massive migration of SPIO-labeled neural stem cells from left striatum into right periventricular region.

Acknowledgements
The Regional Research Universities Program (Biohousing Research Institute) of the Korean Ministry of Education & Human Resources Development(2007) supported this study. We thank Bonnie Hami, MA, Department of Radiology, University Hospital of Cleveland, Ohio, for her editorial assistance in the preparation of this manuscript.
References
1. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000. 97:12846–12851.
2. Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002. 33:2270–2278.
3. Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000. 3:537–544.
4. Savitz SI, Rosenbaum DM, Dinsmore JH, Wechsler LR, Caplan LR. Cell transplantation for stroke. Ann Neurol. 2002. 52:266–275.
5. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001. 344:1450–1460.
6. Lo YK, Yiu Ch, Hu HH, Su MS, Laeuchli SC. Frequency and characteristics of early seizures in Chinese acute stroke. Acta Neurol Scand. 1994. 90:83–85.
7. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003. 34:2258–2263.
8. Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004. 35:952–957.
9. Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental sroke in rat. Proc Natl Acad Sci USA. 2002. 99:16267–16272.
10. Magnitsky S, Watson DJ, Walton RM, Pickup S, Bulte JW, Wolfe JH, et al. In vivo and ex vivo MRI detection of localized and disseminated neural stem cell grafts in the mouse brain. Neuroimage. 2005. 26:744–754.
11. Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004. 21:311–317.
12. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005. 57:874–882.
13. Zhang Z, Ziang Q, Jaing F, Ding G, Zhang R, Wang L, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004. 23:281–287.
14. Frank JA, Miller BR, Arbab AS, Zuwicke H, Jordan EK, Lewis BK, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003. 228:480–487.
15. Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002. 22:899–907.
16. Stroh A, Faber C, Neuberger T, Lorenz P, Sieland K, Jakob PM, et al. In vivo detection limits of magnetically labeled embryonic stem cells in the rat brain using high-field (17.6 T) magnetic resonance imaging. Neuroimage. 2005. 24:635–645.
17. Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Zhang L, et al. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003. 53:259–263.
18. Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA. 1999. 96:15256–15261.
19. Sipe JC, Filippi M, Martino G, Furlan R, Rocca MA, Rovaris M, et al. Method for intracellular magnetic labeling of human mononuclear cells using approved iron contrast agents. Magn Reson Imaging. 1999. 17:1521–1523.
20. de Laquintane BD, Dousset V, Solanilla A, Petry KG, Ripoche J. Iron particle labeling of haematopoietic progenitor cells: an in vitro study. Biosci Rep. 2002. 22:549–554.
21. Arnold LJ Jr, Dagan A, Gutheil J, Kaplan NO. Antineoplastic activity of poly(L-Lysin) with some ascites tumor cells. Proc Natl Acad Sci USA. 1979. 76:3246–3250.
22. Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997. 4:961–968.
23. Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004. 101:11839–11844.
24. Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006. 7:75–84.
25. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001. 32:1005–1011.
26. Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999. 10:1289–1292.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download