Abstract
Objective
The aim of this study was to assess the diagnostic efficacy of integrated PET/CT using fluorodeoxyglucose (FDG) for the differentiation of benign and metastatic adrenal gland lesions in patients with lung cancer and to compare the diagnostic efficacy with the use of PET alone.
Materials and Methods
Sixty-one adrenal lesions (size range, 5-104 mm; mean size, 16 mm) were evaluated retrospectively in 42 lung cancer patients. Both PET images alone and integrated PET/CT images were assessed, respectively, at two-month intervals. PET findings were interpreted as positive if the FDG uptake of adrenal lesions was greater than or equal to that of the liver, and the PET/CT findings were interpreted as positive if an adrenal lesion show attenuation > 10 HU and showed increased FDG uptake. Final diagnoses of adrenal gland lesions were made at clinical follow-up (n = 52) or by a biopsy (n = 9) when available. The diagnostic accuracies of PET and PET/CT for the characterization of adrenal lesions were compared using the McNemar test.
Results
Thirty-five (57%) of the 61 adrenal lesions were metastatic and the remaining 26 lesions were benign. For the depiction of adrenal gland metastasis, the sensitivity, specificity, and accuracy of PET were 74%, 73%, and 74%, respectively, whereas those of integrated PET/CT were 80%, 89%, and 84%, respectively (p values; 0.5, 0.125, and 0.031, respectively).
A drenal metastases are frequently noted in patients with lung cancer (1); however, the majority of adrenal lesions are likely to be benign, even in lung cancer patients (2-5). The reported incidence of adrenal lesions in lung cancer patients, regardless as to whether they are due to metastasis, varies from 4% to 18% in clinical studies (3, 6, 7), and up to 40% of these lesions may be malignant and present as solitary sites of metastasis (8). Therefore, it is essential to differentiate between benign and malignant lesions in lung cancer patients to ensure optimal management. The percutaneous biopsy is the gold standard for confirming the status of adrenal lesions, but is invasive and difficult to perform, and thus frequently leads to complications or study failure (9).
Noninvasive imaging methods have been examined in terms of their abilities to determine adrenal lesion status. Chemical shift MR imaging is found to be useful for differentiating between benign and malignant adrenal lesions (10, 11). CT has proven useful in this context because of its ability to measure attenuation, on both unenhanced images and on delayed contrast-enhanced images (12-14). The use of 18Fluorine-fluorodeoxyglucose (FDG) PET has also shown encouraging results (15-20), but relatively few studies have addressed the usefulness of FDG PET for evaluating adrenal lesions specifically in patients with lung cancer (15, 16, 19).
Recently, the development of integrated PET/CT has allowed functional PET and anatomical CT images to be obtained in one session. The combination of PET and CT data sets using the integrated PET/CT approach is not additive; in fact, it is highly synergistic (21-23). The limited ability of PET to localize accurately lesions, due to a lack of precise anatomical landmarks, has been demonstrated by several studies (24-28). However, several recent studies on the accuracy of PET/CT versus PET have shown that PET/CT helps resolve this problem for ambiguous lesions, especially for anatomy-related lesions (26, 29-33). However, no study has yet addressed the usefulness of integrated PET/CT for differentiating benign and metastatic adrenal lesions in lung cancer patients. Our goal was to assess the diagnostic efficacy of integrated 18F-FDG PET/CT for the differentiation of benign and metastatic adrenal gland lesions in lung cancer patients and to compare these results with the use of 18F-FDG PET alone.
Our institutional review board approved this research study. Patient informed consent was not required for the retrospective study, but written informed consent was obtained from all patients for obtaining the integrated FDG PET/CT study.
From May 2003 to July 2005, we treated 53 lung cancer patients with uncharacterized adrenal lesion(s) detected by contrast-enhanced thoracic (covering from thoracic inlet to middle portion of both kidneys) CT. During the same period, 1,283 new lung cancer patients were registered at our institution. An adrenal gland lesion was considered present when a round or oval lesion (short- and long-axis diameters were within a factor of 1.5 of each other) with a discrete margin was identified in the adrenal gland. When diffuse enlargement without nodule formation was identified, the abnormality was considered hyperplastic and was not included. Patients that had undergone integrated PET/CT were studied before the initiation of chemotherapy or radiation therapy to avoid any potential effects of the therapies as seen by adrenal 18F-FDG uptake. Eleven of the 53 patients were excluded because of a short clinical follow-up period of < 6 months for nine patients and follow-up loss for two patients. Therefore, we included 42 patients (38 men and 4 women; age range 33-77 years; mean ± standard deviation [SD], 62 ± 8.9 years).
Of these 42 patients, 38 underwent PET/CT once. The 38 patients had a total of 52 adrenal gland lesions; 26 patients had a single unilateral adrenal gland lesion, 10 patients had two lesions (one each lesion in each gland) bilaterally, and two patients had three lesions (one in one gland and two in the other gland), also bilaterally. Four patients underwent FDG PET/CT twice at intervals of two months for two patients, of an interval of 11 months in one patient, and an interval of 14 months in the remaining one patient. In one of these four patients, a unilateral lesion was present at the initial study and bilateral lesions were detected at the follow-up study. Therefore, counting the adrenal lesions in each study, nine lesions (six from three patients and three from one patient) from these four patients were also included in the study. Including the nine lesions in these four patients, 61 adrenal lesions in 42 patients were the targets of this study.
Histological analyses showed that the primary lung cancers were adenocarcinomas in 24 patients, squamous cell carcinomas in 15 patients, small cell carcinomas in two patients, and a large cell neuroendocrine carcinoma in one patient. A total of 61 adrenal lesions in 42 patients were evaluated using contrast-enhanced standalone CT reports. All patients underwent integrated PET/CT for the primary tumor characterization and the staging of histologically-proven lung cancer.
The final diagnoses of adrenal lesions were reached by clinical follow-ups (n = 52) or by histopathological examinations (n = 9) of surgical specimens, when available. In the clinical follow-up studies, an adrenal lesion was considered benign if it did not show any change in size for at least six months (mean ± SD for 20 presumed benign adrenal lesions, 14 ± 7 months; range, 6-28 months; median, 12 months). A lesion was considered malignant if it showed an increase or decrease in size after treatment (≥3 mm change in the longest diameter).
All patients fasted for at least six hours before the PET/CT examination, although oral hydration with glucose-free water was allowed. After ensuring a normal blood glucose level in the peripheral blood, patients received an intravenous injection of 370 MBq (10 mCi) of FDG and then rested for approximately 45 minutes before scanning. Scans were acquired using a PET/CT device (Discovery LS; GE Medical Systems, Milwaukee, WI), which consisted of a PET scanner (Advance NXi; GE Medical Systems) and an eight-section CT scanner (LightSpeed Plus; GE Healthcare). The axes of both systems were mechanically aligned such that a patient could be moved from the CT scanner to the PET scanner gantry by moving the examination table by 68 cm.
CT was performed from the head to the pelvic floor according to a standardized protocol using the following settings: 140 kVp; 80 mA; tube rotation time, 0.5 seconds per rotation; pitch, 6; and section thickness, 5 mm (to match the PET section thickness). Patients maintained normal shallow respiration during the acquisition of CT scans. Iodinated contrast material was not administered. Immediately after the unenhanced CT scan, an emission PET scan was performed in the identical transverse field of view. The acquisition time for PET was 5 minutes per table position (each frame). The CT data were resized from a 512 × 512 matrix to a 128 × 128 matrix to match the PET data so that scans could be fused and CT-based transmission maps generated. PET data sets were reconstructed iteratively using an ordered subset expectation maximization algorithm with segmented attenuation correction (two iterations, 28 subsets) using the CT data. Co-registered scans were displayed using eNTEGRA software (GE Medical Systems).
One nuclear medicine physician with 12 years of PET interpretation experience and two years of experience of integrated PET/CT analysis and one radiologist with 16 years of CT experience and two years of experience of integrated PET/CT analysis that were unaware of previous contrast-enhanced CT findings, evaluated the imaging findings twice. Interpretations were performed once with the PET images only and subsequently with the integrated PET/CT images. Decisions on findings were reached by consensus. First, the two observers interpreted the dedicated PET images in a random order without observing the CT component of integrated PET/CT. Two months later, the same nuclear medicine physician and radiologist evaluated the integrated PET/CT images.
When interpreting the dedicated PET images, special attention was given to FDG uptake in the region of the adrenal glands. PET findings were interpreted as positive if the FDG uptake of an adrenal lesion was greater than or equal to that of the liver. PET findings were interpreted as negative if the FDG uptake of the adrenal mass was less than that of the liver. Because the liver was often inhomogeneous in FDG uptake, we chose the visual average (the highest and lowest) of liver uptake for comparison. According to previous studies (15, 17), visual assessment of suspected lesions was just as effective at differentiating active from inactive disease as quantitative analysis using a standardized uptake value (SUV). Therefore, the maximum SUV was not used to differentiate benign and malignant adrenal lesions.
When interpreting the integrated PET/CT images, we also assessed CT component of the adrenal lesions in addition to the PET component of the lesions. The longest diameter of adrenal lesion was measured where it appeared largest on the transverse images. We also recorded the attenuation value of the adrenal lesions. A region of interest, ovoid or circular, covering between one-half to two-thirds of the largest area within the adrenal lesion, was selected. For each of the adrenal lesions, average attenuation values (mean of two measurements) and SDs were obtained.
When lesions had ≤ 10 HU, all adrenal lesions, regardless of the FDG uptake value, were interpreted as benign. When lesions had > 10 HU attenuation value, they were regarded as metastatic with an FDG uptake greater than or equal to that of the liver and as benign with an FDG uptake less than that of the liver. After the image reading sessions, maximum SUVs were recorded if the FDG uptake of the adrenal lesion was considered present at the integrated PET/CT examination. The nodule sizes of the benign and metastatic adrenal lesions were also recorded.
The accuracies, sensitivities, and specificities of PET and PET/CT for the diagnosis of adrenal metastases were assessed using a generalized estimating equation. We regarded each case in which there was agreement between the final diagnosis and the image interpretation as positive, and similarly, each case in which there was disagreement as negative. The diagnostic accuracies of both methods for the diagnosis of adrenal metastasis were compared using the McNemar test. Differences in the maximum SUVs, the nodule sizes, and attenuation values of benign and metastatic adrenal lesions were calculated using the student's t test. P values of < 0.05 were considered to indicate statistical significance. These calculations were performed using a statistical software package (SPSS for Windows; SPSS Inc., Chicago, IL).
The causes, sizes, and maximum SUV values of the false positive and false negative interpretations for both the PET and integrated PET/CT findings were retrospectively assessed.
The sizes of the adrenal lesions on CT scans ranged from 5 to 104 mm in the longest diameter, with a mean of 16 mm and a median of 13 mm.
Thirty-five (57%) of the 61 adrenal lesions eventually were proved to be metastatic adrenal disease, either by surgery (n = 3) or clinical follow-up (n = 32). The remaining 26 adrenal lesions were benign, as determined either by surgery (n = 6) or by at least six months of clinical follow-up (n = 20). Histopathological examinations of the benign adrenal lesions demonstrated the presence of adenomas (n = 5) and endothelial cysts (n = 1). Overall, the mean sizes (± SD) of the 61 adrenal lesions were 16 ± 15 mm (range; 5-104 mm). The mean lesion sizes (± SD) were 13 ± 8 mm for the benign lesions and 18 ± 17 mm for the malignant lesions (p = 0.187) (Table 1). The mean attenuation values (± SD) were 12.4 ± 11.1 for the benign lesions and 29.7 ± 10.9 for the malignant lesions (p < 0.001), and the mean maximum SUV (± SD) values were 1.7 ± 1.84 for the benign lesions and 7.2 ± 5.13 for the malignant lesions (p < 0.001).
The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PET for the detection of metastatic disease were 74% (26 of 35 lesions), 73% (19 of 26 lesions), 79% (26 of 33 lesions), 68% (19 of 28 lesions), and 74% (45 of 61 lesions), respectively. The corresponding values for sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for PET/CT were 80% (28 of 35 lesions), 89% (23 of 26 lesions), 90% (28 of 31 lesions), 77% (23 of 30 lesions), and 84% (51 of 61 lesions), respectively. Thus, the accuracy for PET/CT (84%, 51 of 61 lesions) was significantly higher than that for PET (74%, 45 of 61 lesions) (p = 0.031). However, the values of sensitivity (p = 0.5) and specificity (p = 0.125) for PET/CT were not significantly different from the values for PET.
For the PET images alone, seven false positive interpretations were made for two adenomas that were proven at surgery, for one endothelial cyst that was proven at surgery, for two misinterpretations of hepatic uptakes as adrenal gland uptakes (Fig. 1), and for two misinterpretations of lymph node uptakes as adrenal gland uptakes. For nine false negative interpretations made, the mean size and mean maximum SUV value of the adrenal lesions were 8 mm (range, 5-13 mm) and 1.6 (range, 0-4.5), respectively (Table 1).
For the integrated PET/CT images, three false positive interpretations were made, and these stemmed from two adrenal adenomas (Fig. 2) and one endothelial cyst (Fig. 3) in the adrenal glands. For the seven false negative interpretations made, the mean size and mean maximum SUV value were 7 mm (range, 5-11 mm) and 1.0 (range, 0-2.4), respectively (Table 1).
PET and PET/CT made six discordant interpretations. In four cases, PET produced a false-positive result because FDG uptake in the liver or lymph nodes was misinterpreted as adrenal uptake. In one case in which a patient underwent FDG PET/CT twice with a two-month interval, false-negative results we obtained by PET and by PET/CT at the initial study. However, at the follow-up study, PET continued to show a false-negative result, but PET/CT produced a true-positive result. In this particular case, the adrenal lesion was 6 mm in diameter as determined at the initial study and 11 mm in diameter as determined at the second study. In the remaining single case, a nodule was regarded as benign by PET as the adrenal uptake was interpreted as uptake in the adjacent left kidney, whereas it was correctly interpreted as metastasis by the use of integrated PET/CT (Fig. 4).
CT is the primary diagnostic imaging method for adrenal gland lesion evaluation. Lipid within adenomas causes low attenuation in unenhanced CT images. In addition, adenomas demonstrate rapid washout after intravenous contrast administration (12-14). The sensitivity and specificity of unenhanced CT (at a threshold attenuation of ≤ 10 HU) for distinguishing an adenoma from other diseases has been determined as 79% and 96%, respectively (13). The sensitivity, specificity, and diagnostic accuracy as reported by a dynamic study were 98%, 92%, and 96%, respectively (12).
Signal intensity on T2-weighted and chemical shift imaging using signal intensity reductions between in-phase and opposed-phase MR images were initially investigated in an effort to differentiate benign from malignant lesions. However, benign and malignant lesions were found to overlap considerably in terms of signal intensity (10, 11, 34). Burt et al. (8) reported a false-positive level for unilateral adrenal masses of 67% in patients with operable nonsmall cell lung cancer, i.e., 14 of 21 histologically benign masses were interpreted as malignant masses based on the relative signal strengths of T1- and T2-weighted images.
Unlike CT and MRI, FDG PET intensities are dependent on the glucose metabolism in the malignant lesions. FDG PET in patients with lung cancer and adrenal masses has reported ranges for sensitivities of 93-100%, specificities of 80-100%, and diagnostic accuracies of 92-100% (15-20). However, the limited accuracy of lesion localization using PET alone, due to the lack of precise anatomical landmarks, has been demonstrated by several previous studies (24-28).
Recently, integrated 18F-FDG PET/CT was introduced. This technique can produce directly functional PET and anatomical CT images in one session. Integrated 18F-FDG PET/CT findings are not simply the summation of PET and CT findings; in fact they are the result of a high level of synergism between the two modalities (21-23). Previous studies that have evaluated the accuracy of PET/CT versus PET have shown that rate of ambiguous lesions findings is lower for the use of PET/CT (26, 29-33). In the present study, a statistically significant difference was found between the accuracy of PET and of integrated PET/CT in terms of differentiating benign and malignant adrenal masses in patients with lung cancer.
In this study, the FDG uptake of an adrenal lesion was compared with that of liver, thus an adrenal lesion was interpreted as positive for metastasis if the FDG uptake was greater or equal to that of the liver. However, Bagheri et al. (35) addressed that normal adrenal glands show a wide range of FDG uptake and can demonstrate uptake equal to or slightly greater than liver activity. Recently, Metser et al. (32) suggested that a maximum SUV of > 3.1 usefully differentiates malignant and benign adrenal lesions. However, using a maximum SUV of 3.1 as a cutoff for malignant adrenal nodule detection is only a guideline, and care must be taken when the interpretation is based solely on the SUVs as this approach may potentially lead to a false negative study, especially for small sub-centimeter metastatic adrenal nodules.
Attenuation values of the adrenal lesion were also evaluated on the CT component of the integrated PET/CT images in this study. When lesions had ≤ 10 HU, all adrenal lesions, regardless of the FDG uptake value, were interpreted as benign. When lesions had > 10 HU attenuation value, they were regarded as metastatic with the FDG uptake was greater than in the liver and as benign with the FDG uptake was less than in the liver. Interestingly, image interpretations using an attenuation value combined with FDG uptake were not different with image interpretations only using FDG uptake. Moreover, the sensitivity and specificity of the CT component of the integrated PET/CT (at a threshold attenuation of ≤ 10 HU) for the diagnosis of adrenal metastasis, excluding FDG uptake, were 94% and 35%, respectively. These findings indicate a discrepancy between the current and previously published studies (13), namely, a relatively higher sensitivity and relatively much lower specificity of the unenhanced CT. The discrepancy may be explained by the use of different CT techniques. In this study, attenuation values were measured on the CT component of integrated PET/CT images that were obtained using lower killovoltage (140 kVp) and milliamperage (80 mA) settings compared to the settings employed for conventional CT techniques. Therefore, beam-hardening artifacts more frequently developed and the attenuation values of the adrenal lesions were measured with higher values, especially for the small sized adrenal lesions. We provisionally regarded the CT component of the integrated PET/CT as not useful for the diagnosis of adrenal metastasis.
In contrast to previously published studies for 18F-FDG PET, the present results showed a lower sensitivity, specificity, and diagnostic accuracy. There are several possible reasons to explain these results. First, the adrenal lesions of relatively small sizes were included in this study; the overall mean size (± SD) and median of 61 adrenal lesions were 16 ± 15 mm (range; 5-104 mm) and 13 mm, respectively. In previous studies, the mean sizes of the adrenal lesions were 20 ± 10 mm (range; 5-42 mm) (18), 26 mm (range; 5-54 mm) (15), 24 ± 16 mm (range; 6-110 mm) (32), and 20 mm (range; 5-95 mm) (33), respectively. In this study, CT images were obtained using a multi-detector row CT, which could provide much thinnersection CT images and thus help detect small-sized adrenal gland lesions. Previous studies (16-20) were performed before the development of multi-detector row CT. Second, we included cases of rather long mean follow-up periods, thus, we might have more accurate reference standard values. For example, in this study, 20 benign adrenal lesions had a 14 ± 7 month follow-up period. Additionally, two malignant adrenal lesions, which were erroneously diagnosed as benign by PET (with the use of integrated PET/CT, one lesion was wrongly diagnosed and the other was correctly diagnosed), showed a detectable increase in their sizes at studies performed seven months and 12 months after the initial studies, respectively. In previous studies, their mean follow-up period for making a benign diagnosis for the adrenal lesions could not be identified (15, 18).
The commonly reported causes of false-positive results have been due to pheochromocytomas and benign adenomas (18, 36). There were three cases of false-positive findings shown by integrated PET/CT in the present study. Two cases were due to benign adenomas and one case to an endothelial cyst. The cause of the uptake in the endothelial cyst was unclear.
Commonly reported causes of false-negative results are a small lesion size, necrotic metastases, and metastases from neuroendocrine tumors (18, 37). In the present study, there were seven false-negative integrated PET/CT interpretations, and all were due to small lesion size (range 5-11 mm; mean 7 mm).
One potential limitation of this study is that a biopsy was not performed in all cases. Therefore, we may have missed cases of micrometastasis or slow-growing metastatic lesions in the adrenal gland. Moreover, there might have been metastatic lesions that did not show any size change as chemotherapy hampered lesion growth. This is an inherent drawback in all studies of this type, because not all adrenal lesions can be proven histopathologically. In addition, cortisol, dehydroepiandrosterone, and sex hormone levels were not determined to examine the possibility of stress-induced adrenal activation.
In conclusion, the use of integrated PET/CT using FDG was found to provide 80% sensitivity, 89% specificity, and 84% accuracy for the characterization (metastatic versus benign) of CT-positive adrenal gland lesions in lung cancer patients, and was found to be more accurate than the use of PET alone.
Figures and Tables
Fig. 1
A false-positive interpretation with the use of PET only, but a true negative interpretation with the use of integrated PET/CT in a 57-year-old man with adenocarcinoma in the right upper lobe of the lung.
A. PET demonstrates increased uptake (arrow) in the right adrenal gland area with a maximum standardized uptake value of 3.2. PET alone regarded the uptake as positive.
B. Unenhanced CT scan shows hepatic cysts (arrowheads).
C. On an integrated PET/CT image, the uptake (arrow) falls on the liver, thus enabling a correct interpretation as negative uptake in the right adrenal gland.
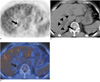
Fig. 2
A false-positive interpretation by the use of both PET and PET/CT in a 33-year-old woman with an adrenal adenoma that was confirmed by surgical excision. The patient also had an adenocarcinoma in the left lower lobe of the lung.
A. PET demonstrates increased uptake (arrow) in the left adrenal gland lesion with a maximum standardized uptake value of 4.6.
B. An unenhanced CT scan shows a 14 mm sized nodule (arrow) in the left adrenal gland.
C. Integrated PET/CT shows high uptake (arrow) in the nodule of the left adrenal gland.
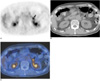
Fig. 3
A false-positive interpretation by the use of both PET and PET/CT in a 56-year-old woman with an endothelial cyst that was confirmed by surgical excision. The patient also had a squamous cell carcinoma in the left lower lobe of the lung.
A. PET demonstrates increased uptake (arrow) in the left adrenal gland lesion with a maximum standardized uptake value of 4.3.
B. An unenhanced CT scan shows a 7 mm sized nodule (arrow) in the left adrenal gland.
C. Integrated FDG PET/CT shows high uptake (arrow) in the nodule of the left adrenal gland.
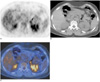
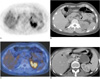
Fig. 4
A false-negative interpretation with the use of PET only, but a true positive interpretation with the use of integrated PET/CT in a 33-year-old woman with an adrenal metastasis confirmed by clinical follow-up. The patient also had adenocarcinoma in the right lower lobe of the lung.
A. PET demonstrates increased uptake (arrow) in the left adrenal gland area with a maximum SUV of 3.5. Uptake was regarded as benign by PET alone because the uptake was interpreted as left kidney uptake.
B. An unenhanced CT scan shows an 11-mm-sized nodule (arrow) in the left adrenal gland.
C. On an integrated PET/CT image, the uptake (arrow) falls on the left adrenal gland, thus enabling a correct interpretation as positive uptake (uptake extent is equal to that of liver).
D. The nodule (arrow) in the left adrenal gland shows an increase in size at a 7-month follow-up CT. Hepatic metastasis is also noted (arrowhead).
References
1. Ettinghausen SE, Burt ME. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. J Clin Oncol. 1991. 9:1462–1466.
2. Hedeland H, Ostberg G, Hokfelt B. On the prevalence of adrenocortical adenomas in an autopsy material in relation to hypertension and diabetes. Acta Med Scand. 1968. 184:211–214.
3. Chapman GS, Kumar D, Redmond J 3rd, Munderloh SH, Gandara DR. Upper abdominal computerized tomography scanning in staging non-small cell lung carcinoma. Cancer. 1984. 54:1541–1543.
4. Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950. 3:74–85.
5. Oliver TW Jr, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984. 153:217–218.
6. Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal thmors: an institutional perspective. Surgery. 1991. 110:1014–1021.
7. Porte HL, Roumilhac D, Graziana JP, Eraldi L, Cordonier C, Puech P, et al. Adrenalectomy for a solitary adrenal metastasis from lung cancer. Ann Thorac Surg. 1998. 65:331–335.
8. Burt M, Heelan RT, Coit D, McCormack PM, Bains MS, Martini N, et al. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. Impact of magnetic resonance imaging. J Thorac Cardiovasc Surg. 1994. 107:584–589.
9. Mody MK, Kazerooni EA, Korobkin M. Percutaneous CT-guided biopsy of adrenal masses: immediate and delayed complications. J Comput Assist Tomogr. 1995. 19:434–439.
10. Namimoto T, Yamashita Y, Mitsuzuki K, Nakayama Y, Makita O, Kadota M, et al. Adrenal masses: quantification of fat content with double-echo chemical shift in-phase and opposed-phase FLASH MR images for differentiation of adrenal adenomas. Radiology. 2001. 218:642–646.
11. Tsushima Y, Ishizaka H, Matsumoto M. Adrenal masses: differentiation with chemical shift, fast low-angle shot MR imaging. Radiology. 1993. 186:705–709.
12. Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002. 222:629–633.
13. Lee MJ, Hahn PF, Papanicolaou N, Egglin TK, Saini S, Mueller PR, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology. 1991. 179:415–418.
14. Pena CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology. 2000. 217:798–802.
15. Kumar R, Xiu Y, Yu JQ, Takalkar A, El-Haddad G, Potenta S, et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004. 45:2058–2062.
16. Erasmus JJ, Patz EF Jr, McAdams HP, Murray JG, Herndon J, Coleman RE, et al. Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol. 1997. 168:1357–1360.
17. Boland GW, Goldberg MA, Lee MJ, Mayo-Smith WW, Dixon J, McNicholas MM, et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995. 194:131–134.
18. Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001. 42:1795–1799.
19. Gupta NC, Graeber GM, Tamim WJ, Rogers JS, Irisari L, Bishop HA. Clinical utility of PET-FDG imaging in differentiation of benign from malignant adrenal masses in lung cancer. Clin Lung Cancer. 2001. 3:59–64.
20. Maurea S, Mainolfi C, Bazzicalupo L, Panico MR, Imparato C, Alfano B, et al. Imaging of adrenal tumors using FDG PET: comparison of benign and malignant lesions. AJR Am J Roentgenol. 1999. 173:25–29.
21. Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000. 41:1369–1379.
22. Townsend DW, Beyer T, Blodgett TM. PET/CT scanners: a hardware approach to image fusion. Semin Nucl Med. 2003. 33:193–204.
23. Townsend DW. A combined PET/CT scanner: the choices. J Nucl Med. 2001. 42:533–534.
24. von Schulthess GK. Positron emission tomography versus positron emission tomography/computed tomography: from
"unclear" to "new-clear" medicine. Mol Imaging Biol. 2004. 6:183–187.
25. Messa C, Bettinardi V, Picchio M, Pelosi E, Landoni C, Gianolli L, et al. PET/CT in diagnostic oncology. Q J Nucl Med Mol Imaging. 2004. 48:66–75.
26. Pelosi E, Messa C, Sironi S, Picchio M, Landoni C, Bettinardi V, et al. Value of integrated PET/CT for lesion localisation in cancer patients: a comparative study. Eur J Nucl Med Mol Imaging. 2004. 31:932–939.
27. Cerfolio RJ, Ojha B, Bryant AS, Raghuveer V, Mountz JM, Bartolucci AA. The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg. 2004. 78:1017–1023.
28. Costa DC, Visvikis D, Crosdale I, Pigden I, Townsend C, Bomanji J, et al. Positron emission and computed X-ray tomography: a coming together. Nucl Med Commun. 2003. 24:351–358.
29. Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003. 348:2500–2507.
30. Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003. 44:1200–1209.
31. Hany TF, Steinert HC, Goerres GW, Buck A, von Schulthess GK. PET diagnostic accuracy: improvement with in-line PET-CT system: initial results. Radiology. 2002. 225:575–581.
32. Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006. 47:32–37.
33. Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy-initial experience. Radiology. 2006. 238:970–997.
34. Haider MA, Ghai S, Jhaveri K, Lockwood G. Chemical shift MR imaging of hyperattenuating (>10 HU) adrenal masses: does it still have a role? Radiology. 2004. 231:711–771.
35. Bagheri B, Maurer AH, Cone L, Doss M, Adler L. Characterization of the normal adrenal gland with 18F-FDG PET/CT. J Nucl Med. 2004. 45:1340–1343.
36. Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. Pheochromocytomas: imaging with 2-[fluorine-18] fluoro-2-deoxy-D-glucose PET. Radiology. 1999. 212:35–41.
37. Erasmus JJ, McAdams HP, Patz EF Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol. 1998. 170:1369–1373.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download