Abstract
Objective
To evaluate the correlations between prostate volumes estimated by transabdominal, transrectal, and three-dimensional US and the factors affecting the differences.
Materials and Methods
The prostate volumes of 94 consecutive patients were measured by both transabdominal and transrectal US. Next, the prostate volumes of 58 other patients was measured by both transrectal and three-dimensional US. We evaluated the degree of correlation and mean difference in each comparison. We also analyzed possible factors affecting the differences, such as the experiences of examiners in transrectal US, bladder volume, and prostate volume.
Results
In the comparison of transabdominal and transrectal US methods, the mean difference was 8.4 ± 10.5 mL and correlation coefficient (r) was 0.775 (p < 0.01). The experienced examiner for the transrectal US method had the highest correlation (r = 0.967) and the significantly smallest difference (5.4 ± 3.9 mL) compared to the other examiners (the beginner and the trained; p < 0.05). Prostate volume measured by transrectal US showed a weak correlation with the difference (r = 0.360, p < 0.05). Bladder volume did not show significant correlation with the difference (r = -0.043, p > 0.05). The comparison between the transrectal and three-dimensional US methods revealed a mean difference of 3.7 ± 3.4 mL and the correlation coefficient was 0.924 for the experienced examiner. Furthermore, no significant difference existed between examiners (p > 0.05). Prostate volume measured by transrectal US showed a positive correlation with the difference for the beginner only (r = 0.405, p < 0.05).
Accurate and reliable measurement of prostate volume is crucial for the management of prostate diseases. It is important not only for benign prostate hypertrophy (BPH), but also in planning nonsurgical therapies of prostate cancers and follow-ups.
So far, ellipsoidal volume calculations, using values measured on transrectal US (TRUS) have been most widely used in prostate volume estimation. Although this method is tolerated in most patients, the transrectal approach is difficult or impossible in some patients (e.g. after Mile's operation). In such cases, the transabdominal or perineal approach should be considered and the former is preferred due to its convenience and accessibility. Previous studies have reported high degree of correlations between the transabdominal US (TAUS) and TRUS methods in prostate volume measurements (1, 2).
Another issue concerning the TRUS volume estimation is the reproducibility of the method. In large hospitals, a patient is usually examined by more than one radiologist over the course of the disease. However, values measured by each examiner may be different, which may confuse clinicians. In this case, volume measurement by three-dimensional (3D) US can be an alternative method. This method has been known to have high reproducibility in other parts (3-6). In the prostate, a study reported a 7% difference in volume assessment depending on methods of prostate boundary segmentation from the 3DUS images (7). Another study revealed that the automated determination of prostate volume showed low variability within a clinically acceptable range (5%) (8).
The purpose of this study is to evaluate the correlations between prostate volumes estimated by transabdominal, transrectal, and 3DUS and the factors affecting the differences.
Ninety-four consecutive patients referred for the TRUS examination (age range, 42-76 years; mean, 58 years) were included in this study over the course of three months. In each patient, the prostate volume and bladder volume were measured by TAUS without additional bladder filling, followed by prostate volume measurement by TRUS. The volume estimation for both approaches was an ellipsoidal volume calculation; the prostate is considered ellipsoidal in shape and the volume (mL) is 0.523 ×width (cm) ×height (cm) ×length (cm) (9). The widths and heights were measured on axial planes and craniocaudal lengths on sagittal planes at their greatest diameter (Fig. 1) An Acuson Sequoia 512 (Siemens Medical Sol. Mountain View, CA) US scanner with either a 3.5 MHz-curved or a 7.5 MHz-endocavitary probe was used for all examinations.
Examinations were randomly allocated to three radiologists, having different degree of experience in performing TRUS: beginner (n = 30), trained (n = 34), and experienced (n = 30). In each examiner group, degree of correlation between prostate volumes measured by TAUS (PVTAUS) and TRUS (PVTRUS) was analyzed. Furthermore, the difference between PVTAUS and PVTRUS (dPV1) was calculated and compared among examiner groups. Degree of correlation between dPV1 and prostate or bladder volume was also analyzed.
In following two months, 58 other consecutive patients (age range 39-72 years; mean, 55 years) were referred for TRUS and the experienced radiologist examined both 2D and 3D TRUS. A Voluson 730 Expert (GE Kretz Ultrasound, Zipf, Austria) US scanner with a 3.3-10 MHz endocavitary 3D probe was used in all examinations. In each patient, routine TRUS examinations were first performed, including the ellipsoidal volume calculations. Next, 3D volume data was acquired and stored digitally. The data were transferred to a separate workstation, and the volumes were measured by virtual organ computer-aided analysis (VOCAL™), which was provided with the software specialized for the scanner (4D View version 5.0; GE Medical Systems Kretztechnik GMbH & Co OHG, Zipf, Austria). In this method, we trace the perimeter of a prostate in a plane, and trace again in another plane rotated from the previous plane by certain degrees (usually 60°). This procedure is repeated until a 360° rotation is complete, followed by the volume calculation by the software (Fig. 2).
Each radiologist (beginner, trained, and experienced) performed 3D volume measurements separately without knowing PVTRUS or prostate volume measured on 3DUS (PV3DUS) by other radiologists. The difference between PVTRUS and PV3DUS (dPV2) and the degree of correlation between these volumes for each radiologist was analyzed.
Statistical analyses were performed using SPSS 10.0 (SPSS, Chicago, IL). We used the Pearson correlation test for the degree of correlation, the student's t-test to compare dPV1, and paired t-test to compare dPV2. In the comparison of PVTAUS and PVTRUS, ANOVA test was done to evaluate possible difference of prostate and bladder volume among examiner groups. Statistical significance was set a priori at p < 0.05.
In the comparison of TAUS and TRUS (Table 1), the mean PVTAUS was 37.3 ± 19.1 mL (13-125 mL), the mean PVTRUS was 40.7 ± 19.8 mL (15-120 mL), and the mean bladder volume was 140.1 ± 92.5 mL (24-472 mL). These values were not significantly different among examiner groups (p > 0.05). PVTAUS and PVTRUS were the same in 4% (n = 4), whereas the PVTAUS was larger in 37% (n = 35) and smaller in 59% (n = 55) than PVTRUS. The mean dPV1 was 8.4 ± 10.5 mL and the correlation coefficient (r) was 0.775 (p < 0.01). The degree of correlation was highest in the experienced group (r = 0.967, p < 0.05) and lowest in the beginner group (r = 0.408, p < 0.01). The mean dPV1 was smallest in the experienced group (5.4 ± 3.9 mL) and largest in the trained group (10.1 ± 10.4 mL). Moreover, the difference was statistically significant between these two groups (p < 0.05). However, the difference was not significant between the other groups (beginner vs. experienced and beginner vs. trained; p > 0.05). The difference was significant between the experienced group and the beginner-trained group (9.7 ± 12.3 mL, p < 0.05) (Table 2).
Prostate volumes measured by TRUS showed a weak, but positive correlation with dPV1 (r = 0.360, p < 0.05). The degree of correlation was higher for the beginner and trained groups (r = 0.554 and 0.546, respectively; p < 0.05), but not significant in the experienced group (r = 0.026). Lastly, bladder volume did not show significant correlation with dPV1 in any group (Table 3).
In the comparison of TRUS and 3DUS (Table 4), the mean PVTRUS was 28.0 ± 13.1 ml (9-70 ml). Each radiologist yielded PV3DUS values that were highly correlated with PVTRUS (r = 0.909 in the beginner, 0.923 in the trained, and 0.924 in the experienced). The mean dPV2 was largest in the beginner (4.7 ± 4.1 mL), but did not show significant difference between examiner groups (p > 0.05, Table 2). PVTRUS showed a positive correlation with dPV2 only in the beginner (r = 0.405, p < 0.05) (Table 3).
There have been studies working on the correlation of TAUS and TRUS in the prostate volume measurement (1, 2, 10, 11). One study reported that the Pearson correlation coefficient was 0.84 and mean difference was 1.0 ± 1.4 mL (1). Our result showed a larger difference and a lower degree of correlation, which may be due to the examiners' experiences in TRUS. In the former study, TRUS was performed by three examiners who were not classified by the experience in TRUS, and the TAUS was performed by one examiner. No significant difference in prostate volumes by transabdominal and transrectal US were noted between observers for this study. In our study, both TAUS and TRUS were performed by three examiners with varying degrees of experiences in the TRUS with the experienced examiner showing the highest degree of correlation and the smallest difference. Another prior study reported that, although good agreement between the two methods was found, a wide variation in prostatic volume was found between observers and the two methods in the individual patients (2).
We believe that the reason for experience being a factor which affects the correlation of TRUS to TAUS is primarily related with the determination of the caudal end of the prostate on TAUS. A poor sonic window for the TAUS method limits the view of the caudal part of the prostate (Fig. 1A). In this situation, the examiner experienced in TRUS can determine the caudal end more accurately by 'imaging' the view on TRUS based on the shape of the prostate. This is considered a difficult task for less experienced examiners in TRUS resulting in over- or underestimated values. In a previous study comparing prostate dimensions measured by TAUS and TRUS, the craniocaudal length showed the least degree of agreement (25.5%) compared to the transverse (31.6%) and AP diameter (33.1%) (10). Another explanation for these differences in prostate volume estimation comes from our teaching experience in TRUS, where beginners tend to include the membranous urethra in the caudal part of the prostate, resulting in longer craniocaudal length (Fig. 1B). Our study indicates that the PVTRUS was larger than PVTAUS in 59% of the cases.
Although bladder filling is essential for pelvic organ examinations by TAUS, our result showed that it had little influence on prostate volume measurement. It is evident that good sonic windows provide the background for the accurate measurement of volumes, but an overdistended bladder may also distort and displace the prostate. As far as the prostate is within the field of view on TAUS, additional bladder filling is not helpful in the measurement of the prostate volume.
The prostate volume itself had a weak positive correlation with the differences in the comparison of PVTAUS and PVTRUS. This correlation was more pronounced in beginner and trained groups; however, not significant in the experienced group. The comparison of PVTRUS and PV3DUS revealed that only the beginner group showed a significant correlation. Errors in the prostate volume estimation by TRUS have been known to be volume-dependent (12, 13). For TAUS, a larger prostate may make the measurement of dimensions difficult, especially the craniocaudal length. Besides, in the ellipsoidal volume calculation used in both approaches, small differences in the diameter may cause large errors in the calculated volume. These multiple factors may be responsible for the correlation.
The ellipsoidal volume calculation is used in both TAUS and TRUS volume measurements, but the method is only an estimation of real volume. Volume measurement by 3DUS is not a process of estimation; it actually measures volumes even in irregularly shaped structures. The volume measurement method first introduced by 3DUS, involves tracing the perimeter of the structure in multiple parallel planes (14). The narrower the increments between the planes, the more accurate volumes can be calculated. A rotational tracing method was recently introduced, in which the 360° circumference surrounding a structure is divided into precise increments by the software. This technique is known as virtual organ computer-aided analysis (VOCAL™) and has been reported to be reproducible (7, 15, 16).
According to an early study on 3DUS of the prostate, a 20% difference was observed between the PV3DUS and PVTRUS methods (17). This difference may be insignificant in many patients, however, may not be acceptable in calculating PSA densities and planning the management and the follow-up of prostate cancers. In our results, the differences were smaller (13%), which may be explained by technical improvement of US hardwares and 3DUS technology since the early era.
Because a gold standard is not available for prostate volume measurements in patients, reproducibility is more important than the absolute accuracy. A previous study reported 10% of the variation in repeatedly measured prostate volume by TRUS between two observers and 5% for one observer (18). Although a high level of reproducibility for TRUS volume measurements can be expected when performed by experienced observers, interexaminer variability is problematic in examiners with less experience in TRUS (19, 20). Tong et al. (21) performed a study to evaluate the intra- and inter-observer variability and reliability of prostate volume measurement using 2D and 3D TRUS methods. They reported that the 3D TRUS methods had a much lower variability and higher reliability than 2D TRUS methods. However, they did not work on the relationship of experience in TRUS with 3DUS volume measurement. Our study proved that 3DUS volume measurement was not dependent on examiners' experience in TRUS.
Considering our results, the 3DUS volume calculation can be considered as the most reliable method for prostate volume measurement on US. It is especially useful in large hospitals, which have many examiners of different skill levels examining the prostate volume on TRUS are working. However, it should be also taken into account that 3DUS volume measurements require additional time and needs additional high-priced equipment and software. Our study also proved that PVTRUS was well correlated with PV3DUS. Therefore volume estimation by 2D TRUS is also a reliable method if performed by experienced examiners.
We did not compare the volumes by these three methods in each patient, which is one of limitations of this study. Such a study was practically difficult because patients had to suffer long examination time and additional discomfort. Another limitation in this study is the absence of a gold standard, that is, a real volume assessment of the prostate.
In conclusion, in the prostate volume estimation by US, experience in TRUS is important in the correlation with TAUS, but not with 3DUS. Less experienced examiners can be also affected by prostate volume itself. Bladder volume is not an important factor in the prostate volume estimation by TAUS.
Figures and Tables
Fig. 1
Measurement of craniocaudal length of prostate on US. Craniocaudal length of prostate (between crosses) is measured on transabdominal US (A) and transrectal US (B) at mid-sagittal plane.
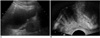
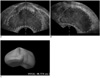
Fig. 2
Prostate volume measurement by 3DUS (VOCAL™).
A. In any plane, trace outer margin of prostate (dotted line).
B. Image rotates along axis (between arrowheads) by 60°, then outline again.
C. Steps A and B are repeated until rotation reaches 360°, followed by 3D image of prostate in surface rendering mode; calculated volumes are displayed.
References
1. Huang Foen Chung JW, de Vries SH, Raaijmakers R, Postma R, Bosch JL, van Mastrigt R. Prostate volume ultrasonography: the influence of transabdominal versus transrectal approach, device type and operator. Eur Urol. 2004. 46:352–356.
2. Styles RA, Neal DE, Powell PH. Reproducibility of measurement of prostatic volume by ultrasound. Comparison of transrectal and transabdominal methods. Eur Urol. 1988. 14:266–269.
3. Gilja OH, Thune N, Matre K, Hausken T, Odegaard S, Berstad A. In vitro evaluation of three-dimensional ultrasonography in volume estimation of abdominal organs. Ultrasound Med Biol. 1994. 20:157–165.
4. Chang FM, Hsu KF, Ko HC, Yao BL, Chang CH, You CH, et al. Three-dimensional ultrasound assessment of fetal liver volume in normal pregnancy: a comparison of reproducibility with two-dimensional ultrasound and a search for a volume constant. Ultrasound Med Biol. 1997. 23:381–389.
5. Riccabona M, Nelson TR, Pretorius DH, Davidson TE. In vivo three-dimensional sonographic measurement of organ volume: validation in the urinary bladder. J Ultrasound Med. 1996. 15:627–632.
6. Riccabona M, Nelson TR, Pretorius DH, Davidson TE. Distance and volume measurement using three-dimensional ultrasonography. J Ultrasound Med. 1995. 14:881–886.
7. Hu N, Downey DB, Fenster A, Ladak HM. Prostate boundary segmentation from 3D ultrasound images. Med Phys. 2003. 30:1648–1659.
8. Aarnink RG, De La Rosette JJ, Debruyne FM, Wijkstra H. Reproducibility of prostate volume measurements from transrectal ultrasonography by an automated and a manual technique. Br J Urol. 1996. 78:219–223.
9. Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol. 1991. 145:984–987.
10. Blanc M, Sacrini A, Avogadro A, Gattamorta M, Lazzerini F, Gattoni F, et al. Prostatic volume: suprapubic versus transrectal ultrasonography in the control of benign prostatic hyperplasia. Radiol Med (Torino). 1998. 95:182–187.
11. Kobayashi T, Kawahara T, Nishizawa K, Ogura K, Mitsumori K, Ide Y. Value of prostate volume measurement using transabdominal ultrasonography for the improvement of prostate-specific antigen-based cancer detection. Int J Urol. 2005. 12:881–885.
12. Matthews GJ, Motta J, Fracehia JA. The accuracy of transrectal ultrasound prostate volume estimation: clinical correlations. J Clin Ultrasound. 1996. 24:501–505.
13. Loeb S, Han M, Roehl KA, Antenor JA, Catalona WJ. Accuracy of prostate weight estimation by digital rectal examination versus transrectal ultrasonography. J Urol. 2005. 173:63–65.
14. Sehgal CM, Broderick GA, Whittington R, Gorniak RJ, Arger PH. Three-dimensional US and volumetric assessment of the prostate. Radiology. 1994. 192:274–278.
15. Wang Y, Cardinal HN, Downey DB, Fenster A. Semiautomatic three-dimensional segmentation of the prostate using two-dimensional ultrasound images. Med Phys. 2003. 30:887–897.
16. Hodge AC, Fenster A, Downey DB, Ladak HM. Prostate boundary segmentation from ultrasound images using 2D active shape models: optimisation and extension to 3D. Comput Methods Programs Biomed. 2006. 84:99–113.
17. Hamper UM, Trapanotto V, DeJong MR, Sheth S, Caskey CI. Three-dimensional US of the prostate: early experience. Radiology. 1999. 212:719–723.
18. Bates TS, Reynard JM, Peters TJ, Gingell JC. Determination of prostatic volume with transrectal ultrasound: A study of intraobserver and interobserver variation. J Urol. 1996. 155:1299–1300.
19. Sech S, Montoya J, Girman CJ, Rhodes T, Roehrborn CG. Interexaminer reliability of transrectal ultrasound for estimating prostate volume. J Urol. 2001. 166:125–129.
20. Collins GN, Raab GM, Hehir M, King B, Garraway WM. Reproducibility and observer variability of transrectal ultrasound measurements of prostatic volume. Ultrasound Med Biol. 1995. 21:1101–1105.
21. Tong S, Cardinal HN, McLoughlin RF, Downey DB, Fenster A. Intra- and inter-observer variability and reliability of prostate volume measurement via two-dimensional and three-dimensional ultrasound imaging. Ultrasound Med Biol. 1998. 24:673–681.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download