Abstract
Objective
To investigate the relationship between the perfusion CT features and the clinicopathologically determined prognostic factors in advanced gastric cancer cases.
Materials and Methods
A perfusion CT was performed on 31 patients with gastric cancer one week before surgery using a 16-channel multi-detector CT (MDCT) instrument. The data were analyzed with commercially available software to calculate tumor blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability surface (PS). The microvessel density (MVD), was evaluated by immunohistochemical staining of the surgical specimens with anti- CD34. All of the findings were analyzed prospectively and correlated with the clinicopathological findings, which included histological grading, presence of lymph node metastasis, serosal involvement, distant metastasis, tumor, node, metastasis (TNM) staging, and MVD. The statistical analyses used included the Student's t-test and the Spearman rank correlation were performed in SPSS 11.5.
Results
The mean perfusion values and MVD for tumors were as follows: BF (48.14±16.46 ml/100 g/min), BV (6.70±2.95 ml/100 g), MTT (11.75±4.02 s), PS (14.17±5.23 ml/100 g/min) and MVD (41.7±11.53). Moreover, a significant difference in the PS values was found between patients with or without lymphatic involvement (p = 0.038), as well as with different histological grades (p = 0.04) and TNM stagings (p = 0.026). However, BF, BV, MTT, and MVD of gastric cancer revealed no significant relationship with the clinicopathological findings described above (p > 0.05).
With a mortality rate as high as 25 in 100,000, the prognosis of gastric cancer in China is very poor (1), and is closely related to TNM (tumor, node, metastasis) staging, histological classification and differentiation, lymphatic metastasis, as well as serosal infiltration (2, 3). Angiogenesis was originally reported by Folkman (4) in 1971, as the process of new blood vessel development, and reflects the generation of a new blood supply into tumor tissue that is now regarded as a prognostic indicator for several tumors. Generally, a tumor is measured by its microvessel density (MVD), which is a somewhat invasive technique, depending on the availability of postoperative tissue or adequate biopsy material (5).
Though multi-detector CT (MDCT) has been used for the staging of gastric carcinoma, the morphological features could not reflect the physiologic tissue parameters. Consequently, no adequate imaging modality has been reliable enough to predict the prognosis or to assist in the selection of the right patients for extended surgical procedures and for trials of adjuvant chemotherapy. The advent of spiral CT systems in the 1990s enabled perfusion scans to be performed with MDCT, thus broadening the technique's availability, and permitting the measurement of the tumor vascular physiology in the brain, lung, liver, neck, and breast (6-8). The method could also be useful for assessing risk-stratification and therapeutic monitoring (9). Currently, the major clinical applications for perfusion CTs include acute stroke and oncology cases, such as tumor grading in cerebral glioma and lymphoma, and the prediction of tumor response to radiotherapy (6, 10). The relationship between the perfusion values and the prognosis of gastric cancer has, to the best of our knowledge, not been reported. The purpose of this study was to evaluate the relationship between perfusion CT and clinicopathological features of gastric adenocarcinoma and its potential as a novel prognostic predictor.
Thirty one cases of pathologically proven gastric cancer occurring from March 2002 to March 2005 were prospectively included in this study. The patient records and the pertinent clinical data are shown in Table 1 and include tumor size (greatest diameter), pathological TNM (pTNM) staging (UICC 1997) and WHO histological grading (11) as described: well differentiated = an adenocarcinoma with well-developed glands and often resembling a metaplastic intestinal epithelium; moderately differentiated = an adenocarcinoma intermediate between the well differentiated and poorly differentiated; poorly differentiated = an adenocarcinoma composed of highly irregular glands that are recognized with difficulty, or single cells that remain isolated or are arranged in small to large clusters with mucin secretions or acinar structures.
A CT perfusion was approved by the Hospital Ethics Committee and a patient consent was obtained before imaging. All tumors were resected via a surgical procedure at one week after MDCT scanning (GE-Lightspeed, GE Medical Systems, Milwaukee, WI).
Following a 1,000-1,200 ml oral dose of water, and a 20 mg intravenous injection of Scopolamine (Boehringer Ingelheim, Germany), a perfusion scan was performed in a 16-section MDCT cine period lasting 60sec. The patients were instructed to practice a gentle breathing technique while scanning, and were fixed with an abdominal belt. The lesion was localized on the non-enhanced CT scan with a 2 cm tumor region of interest defined by an experienced radiologist. The dynamic scan was acquired at this level, performed at a static table position during an intravenous bolus of 50 ml in nonionic iodinated contrast enhance medium (Iopromide, Ultravist300; Schering, Berlin, Germany) via the antecubital vein at 4-5 ml/s. The following parameters were used: 10 s for the scan delay from the start of injection; 5 mm slice thickness (reconstruction slice thicknesses were 5 mm and 10 mm); 1 s gantry rotation period with a 1 s temporal resolution; 120 kV, 100 mA and a 60 sec transverse data acquisition period. Next, the data were transferred to a workstation (Advantage Windows 4.0; perfusion 3.0; GE Medical Systems) and analyzed using commercial software. The advantages of the analytical method included a deconvolution for perfusion and blood volume, which demonstrated good temporal resolution and high spatial resolution, while high image noise and limited volume coverage appeared to be its disadvantages.
The abdominal artery was used as a partial-volume averaging correction. Each region of interest, drawn over the tumor by a radiologist section by section, was as large as possible to reduce noise (> 50 pixels) and to almost completely cover the whole lesion, with care to exclude peripheral fat and necrotic area. Maps of tumor blood flow (BF) (ml/100 g/min), blood volume (BV) (ml/100 g), mean transit time (MTT) (sec), and permeability surface (PS) (ml/100 g/min) were calculated automatically for all four sections (with reconstruction slice thickness 5 mm) available for each patient. The same process was repeated in the sections where reconstruction of slice thickness was 10 mm. Moreover, the mean values were recorded for further study.
All patients were evaluated pathologically for histologic diagnosis and MVD counting. The MVD was estimated by immunohistochemical staining of the surgical specimens with anti-CD34 monoclonal antibody (Dako, Copenhagen Denmark) (12).
The criteria for vessel counting were previously established by Weidner et al. (13). Any single brownstaining endothelial cell or small clusters of brown-staining endothelial cells, with or without a lumen, are clearly separated from its adjacent microvessels, tumor cells, and other connective tissue elements considered as being individual vessels. Vessels of a caliber larger than the approximately eight red blood cells and vessels with a thick muscular wall were excluded from the final counting. The MVD counting was performed by a single pathologist.
After screening the areas with intense neovascularized spots in a low power field (40), the microvessels in the area with the highest number of discrete microvessels were counted in a 400x field. Moreover, three separate areas of intense neovascularization were assessed for each sample and the mean MVD was calculated for each tumor evaluated.
The BV, BF, MTT, PS, and MVDs are expressed as the mean±standard deviation (SD) and the data were subjected to a Kolmogorov-Smirnov normality test. All findings were prospectively analyzed and were found to be correlated with the clinicopathological results (histological grading, presence of lymph node metastasis, serosal involvement, TNM staging, and MVD). The statistical analyses (t-test and Spearman rank correlation) were calculated in SPSS 11.5 (SPSS Inc., Chicago, IL). For the two-tailed tests, p-values less than 0.05 were considered statistically significant.
The perfusion parameters for gastric cancer in 31 patients (with reconstruction slice thicknesses of 5 and 10 mm) were compared, and revealed no significant differences (p > 0.3). In this study, the 5 mm reconstruction slice thickness data was used for all statistical analyses.
The mean perfusion values and MVD for tumors are as follows: BF (48.14±16.46 ml/100 g/min), BV (6.70±2.95 ml/100 g), MTT (11.75±4.02 sec), and PS (14.17±5.23 ml/100 g/min) and MVD (41.7±11.53/400x field). Moreover, the relationship between values and different histological grades, serosal involvement, distant metastasis, or lymphatic metastasis, and pTNM are detailed in Tables 2, 3, 4, 5. A significant difference exists for the PS value between patients with or without lymphatic involvement (p = 0.038), as well as among different histological grades (p = 0.04) and TNM staging (p = 0.026). However, the BF, BV, MTT, and MVD of gastric cancer revealed no significant relationship with any clinicopathological features (p > 0.05) (Figs. 1, 2).
Currently, a perfusion CT is calculated by the deconvolution approach, which is based on the theory that immediate enhancement of the tumor is largely due to the presence of the contrast media within the intravascular space and its first-pass to the extravascular space. With the leakage to the extravascular space, the enhancement of the tumors are caused by the contrast media in both the intrasvascular and extravascular space (10). The deconvolution model has the ability to tolerate greater image noise, is therefore rather well suited to abdominal scans, and is the preferred method for the measurement of low levels of perfusion, which are applicable to gastrocarcinoma (14).
No consensus has been reached regarding optimal scan techniques in the published literature. The first item of controversy and debate is the acquisition time. The majority of the injected contrast media remains intravascular within 40 s (it is also affected by cardiac output and central blood volume); however, a much greater proportion later passes into the extravascular space. Eventually, equilibrium is reached and the blood returns into the intravascular space. The duration of this process lasts around 2 to 4 min (15-17). A perfusion CT scan was performed prospectively in 10 patients with histologicallyproven colorectal cancer using different acquisition times (45, 60, and 130 s) by Goh et al. (18). No significant difference was found for BF, BV, or MTT values between any acquisition times; however, significant differences in PS were found between 45 sec and 65 or 130 sec. By measuring the attenuation change within the input artery and tumor, an IRF (input response function) curve can be derived. The peak of this curve is regarded as BF, while BV is represented by the area under the curve. As a result, different acquisition times do not drastically affect these three values since the shape of the curve is fixed. Although the radiation dose in a perfusion CT scan is around 700 mGy (70% of abdominal CT), the extra 2 min dose may be harmful to the patient and efforts should be taken to minimize the dose. Furthermore, a long acquisition time can lead to respiratory motion in an abdominal scan. When taking all of these elements into account, we determined that the 60 sec acquisition time be the most appropriate for our study.
The 60 sec acquisition time would be very difficult to diminish respiratory artifacts. It is most unlikely that the patients will suspend respiration for 60 sec. Furthermore, patients who suspend their breathing will often exhale, which will lead to more motion artifacts and the temptation to take a deep breath when experiencing the "hot flush" associated with a rapid bolus of contrast medium will interrupt the perfusion scanning. Therefore, a quiet respiration was requested during the scan, with patients in the prone position and fixed with a waistline to reduce the extension of respiration (14).
Pathological TNM staging, histological grading, serosal involvement, lymphatic metastasis, and MVD have been identified as important predictors of the prognosis resulting from gastric cancer in our study (19-20). Moreover, pTNM is the conventional prognostic index as it reflects the depth of invasion as well as lymphatic or distal metastasis (3). Tumor outcome is determined by tumor size, extent, stage, and biological behavior and is largely described according to its histological grade. Recent studies have acknowledged that the lymphatic metastasis status is critical in the prognosis of gastric cancer. The mortality of patients with lymphatic metastasis is 3.92x greater than patients without lymphatic metastasis. Angiogenesis is a highly complex phenomenon that is essential for the growth of solid tumors, hence permitting rapid tumor growth and increased potential for tumor metastasis. Furthermore, angiogenesis is a significant predictor of prognosis and hematogenous metastasis in patients with gastric cancer. MVD is currently applied as an indicator of tumor angiogenesis, and has further been demonstrated that, in some tumors, up to 15% of tumor blood vessels demonstrate the presence of cancer cells in the lumen (21). It is proposed that these small, leaky vessels allow tumor cells to reach the circulatory system, thus increasing the probability of metastasis. The results of a multiple elements analysis in breast cancer, indicated that MVD plays a more important role in predicting invasion and metastases than other clinical indicators. Also, several studies have shown a positive statistical correlation between MVD and metastasis in other solid tumors such as in the lung, uterus, cervix, and prostate. However, the relationship is controversial in gastric cancer (5, 22-24).
According to the results of this study, there is a significant difference for the PS value between patients with and without lymphatic involvement. Moreover, there is also a significant difference between different histological grades and TNM stages, while BF, BV, MTT, and MVD revealed no significant correlation with clinicopathological findings for gastric cancer.
Permeability surface represents the transmission rate of contrast media from capillary endothelium to interstitial space, reflects the integrity of endothelial cells and permeability of vessels. It is known that tumor capillaries, in general, have wide inter-endothelial junctions, a large number of fenestrate and transendocannels, and discontinuous or absent basement membrane. Compared to normal vessels, they are more easily penetrated by large particles, including tumor cells. This whole process often happens along with an inflammatory reaction that accelerates permeability changes (22, 25). Previous reports have put too much emphasis on blood flow of the tumor rather than the functional differences compared to normal vesselscapillary permeability, which appears to be of value in grading of cerebral glioma and lung cancer (13, 26). We believe PS to be an indicator of prognosis in gastric cancer, warranting further study in larger cohort of patients.
There is no significant difference in the BF, BV, MTT and MVD between patients with and without lymphatic involvement, but also with different histological grades and TNM staging. The reasons for this may be as follows: First, this may be due to the small size of the study population, which contained many patients with poorly differentiated carcinoma, which perhaps causes some statistical bias. Second, elevated tissue pressure present at the center of such tumors have caused the lumen and resulted in the underestimation of the MVD (3). Third, it has been suggested by Sahani et al. (10) that angiogenesis stimulates the opening of significant numbers of arteriovenous shunts, rather than producing a new vascular supply (10). Anything above may affect the result of BF, BV and MTT values. Moreover, different tumors have different pathological features. Regional cerebral blood volume is closely related to the grading of glioma (27). Fourth, tumor vessels are structurally and functionally abnormal, with uneven diameters, increased length and tortuosity, multiple arteriovenous shunts, and loss of physiological regulation of blood flow. The distribution of tumor vessels is uneven, with a large amount in the vascular area and a small amount in necrotic region. Because MVD is a direct technique has a significant shortcoming as it depends on the availability of postoperative tissue or biopsy materials. Also, different areas will present different densities. Additionally, a gastric ulcer is associated with cancer, thus complicating accurate calculation (28). Fifth, there is the possibility that MVD acts as an indicator of angiogenesis correlates hematogeneous metastasis.
Several limitations exist in our study. First, as the size of the study population was too small, no estimation of the absolute value of PS data could be provided for under a clinical analysis. Second, too many patients with poorly differentiated carcinoma lead to statistic bias. Third, the lack of control group in the study is another limitation, the normal gastric wall is more likely to be too thin for us to achieve perfusion CT values. Fourth, a respiratory artifact is something hard to overcome, especially for a 60 sec free breathing scan. It may interfere with the results. Fifth, extraradiation dose of CT perfusion is harmful and we should minimize it. It has been reported that MRI could aid in optimizing treatments, categorizing lesions, and influencing patient care in brain tumors (26), which might be a hint for the futher investigation in gastrointestinal system. There is still a long way to go.
In summary, perfusion CT is a reproducible, objective, and feasible imaging strategy for the evaluation of oncology. Furthermore, the analysis of perfusion CT in gastric cancer (especially for the PS value), might be a helpful prognostic indicator, or as being useful for predicting the response to clinical treatment.
Figures and Tables
Fig. 1
Poorly differentiated gastric cancer.
A. Cross section before contrast administration revealed protruding lesion classified as Borrmann I. Regions of interest have been placed between abdominal aorta and lesion.
B. Corresponding time density curves show arterial and tumor attenuation change with time.
C. Image calculating blood volume with mean value, 6.71 ml/100 g.
D. Image calculating blood flow with mean value, 73.64 ml/100 g/min.
E. Image calculating mean transit time with mean value, 10.45 sec.
F. Those values of lesion were rather lower; permeability surface (mean, 18.49 ml/100 g/min) appeared to be rather high.
G. This patient had low microvessel density (mean, 21/*400 field).
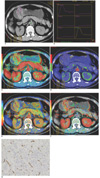
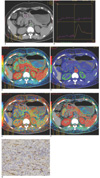
Fig. 2
Poorly differentiated gastric cancer.
A. Cross section prior to contrast administration revealed ulcerative lesion at antrum classified as Borrmann II.
B. Corresponding time density curves reveals arterial and tumor attenuation change with time.
C. Image calculating blood volume with mean value, 4.59 ml/100 g.
D. Image calculating blood flow with mean value, 100.45 ml/100 g/min.
E. Image calculating mean transit time with mean value, 4.1 sec.
F. Lesion values were lower; permeability surface (mean, 21.93 ml/100 g/min) was relatively high.
G. Patient had high microvessel density (mean, 61/*400 field).
References
1. Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, et al. Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Zhonghua Zhong Liu Za Zhi. 2004. 26:4–8.
2. Ahn MJ, Jang SJ, Park YW, Choi JH, Oh HS, Lee CB, et al. Clinical prognostic values of vascular endothelial growth factor, MVD and p53 expression in esophageal carcinomas. J Korean Med Sci. 2002. 17:201–207.
3. Tenderenda M, Rutkowski P, Jesionek-kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res. 2001. 7:129–134.
4. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971. 285:1182–1186.
5. Tuncbilek N, Karakas HM, Altaner S. Dynamic MRI in indirect estimation of MVD, histologic grade, and prognosis in colorectal adenocarcinomas. Abdom Imaging. 2004. 29:166–172.
6. Roberts HC, Roberts TP, Lee TY, Dillon W. Dynamic, contrast-enhanced CT of human brain tumors: quantitative assessment of blood volume, blood flow, and microvascular permeability. AJNR Am J Neuroradiol. 2002. 23:828–832.
7. Blomley MJ, Coulden R, Bufkin C, Lipton MJ, Dawson P. Contrast bolus dynamic computed tomography for the measurement of solid organ perfusion. Invest Radiol. 1993. 28:suppl 5. S72–S77.
8. Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997. 205:471–478.
9. Dugdale PE, Miles KA. Hepatic metastases: the value of quantitative assessment of contrast enhancement on computed tomography. Eur J Radiol. 1999. 30:206–213.
10. Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005. 234:785–792.
11. Hamilton SR. Pathology and genetics of tumors of the digestive system. 2000. Lyon: IARC press;46–48.
12. Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, et al. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004. 213:73–82.
13. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J. 1991. 324:1–8.
14. Miles KA. Perfusion CT for the assessment of tumor vascularity: which protocol? Br J Radiol. 2003. 76:S36–S42.
15. Hoeffner EG, Case I, Jain R, Gujar SK, Shah GV, Deveikis JP, et al. Cerebral perfusion CT: technique and clinical applications. Radiology. 2004. 231:632–644.
16. Eastwood JD, Lev MH, Provenzale JM. Perfusion CT with iodinated contrast material. AJR Am J Roentgenol. 2003. 180:3–12.
17. Harvey CJ, Blomley MJ, Dawson P, Morgan JA, Dooher A, Deponte J, et al. Functional CT imaging of the acute hyperemic response to radiation therapy of the prostate gland: early experience. J Comput Assist Tomogr. 2001. 25:43–49.
18. Goh V, Halligan S, Hugill JA, Gartner L, Bartram CI. Quantitative colorectal cancer perfusion measurement using dynamic contrast-enhanced multidetector -row CT. J Comput Assist Tomogr. 2005. 29:59–63.
19. Setala LP, Kosma VM, Marin S, Lipponen PK, Eskelinen MJ, Syrjanen KJ, et al. Prognostic factors in gastric cnacer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer. 1996. 74:766–772.
20. Gabbert HE, Meier S, Gerharz CD, Hommel G. Tumor cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int J Cancer. 1992. 50:202–207.
21. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000. 407:249–257.
22. Raghunand N, Gatenby RA, Gillies RJ. Microenviromental and cellular consequences of altered blood flow in tumors. Br J Radiol. 2003. 76:S11–S22.
23. Kuszyk BS, Corl FM, Franano FN, Bluemke DA, Hofmann LV, Fortman BJ, et al. Tumor transport physiology: implications for imaging and imaging-guided therapy. AJR Am J Roentgenol. 2001. 177:747–753.
24. Li ZP, Meng QF, Sun CH, Xu DS, Fan M, Yang XF, et al. Tumor angiogenesis and dynamic CT in colorectal carcinoma: radiologic-pathologic correlation. World J Gastroenterol. 2005. 11:1287–1291.
25. Brix G, Bahner ML, Hoffmann U, Horvath A, Schreiber W. Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT-initial experience. Radiology. 1999. 210:269–276.
26. Schmainda KM, Rand SD, Joseph AM, Lund R, Ward BD, Pathak AP, et al. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004. 25:1524–1532.
27. Shin JH, Lee HK, Kwun BD, Kim JS, Kang W, Choi CG, et al. Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. AJR Am J Roentgenol. 2002. 179:783–789.
28. Abdalla SA, Behzad F, Bsharah S, Kumar S, Amini SK, O'Dwyer ST, et al. Prognostic relevance of MVD in colorectal tumors. Oncol Rep. 1999. 6:839–842.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download