Abstract
Objective
To compare the diagnostic performances of conventional ultrasound (US) and US elastography for the differentiation of nonpalpable breast masses, and to evaluate whether elastography is helpful at reducing the number of benign biopsies, using histological analysis as a reference standard.
Materials and Methods
Conventional US and real-time elastographic images were obtained for 100 women who had been scheduled for a US-guided core biopsy of 100 nonpalpable breast masses (83 benign, 17 malignant). Two experienced radiologists unaware of the biopsy and clinical findings analyzed conventional US and elastographic images by consensus, and classified lesions based on degree of suspicion regarding the probability of malignancy. Results were evaluated by receiver operating characteristic curve analysis. In addition, the authors investigated whether a subset of lesions was categorized as suspicious by conventional US, but as benign by elastography.
Breast ultrasonography (US) can be used to help differentiate benign and malignant solid masses. Stavros et al. (1) reported 98% sensitivity, 68% specificity, and 99% negative predictive value using a classification model based on criteria which included lesion shape, orientation, margin, echogenicity, and acoustic transmission (1-3). In addition to morphological criteria, mass compressibility during US scanning has also been employed to differentiate breast lesions. During compression with a transducer, soft breast lesions, such as, cysts or hamartomas, flatten more so than stiffer solid masses (4, 5). Krouskop et al. (6) reported that fat, normal glandular tissue, fibrous tissue, ductal carcinoma in situ, and infiltrating ductal carcinoma of the breast had different elastic moduli at different strain levels, and in particular, found that infiltrating ductal carcinomas were much stiffer than other breast tissues. Based on these viscoelastic characteristics, US elastography, whereby US echo signals are obtained from tissues before and after compression and then converted to displacement distribution images, was rapidly developed (7-9). By measuring tissue displacements, elastography is able to provide objective information on tissue stiffness. During early clinical trials, elastography was found to have the potential to differentiate benign and malignant solid breast masses (10, 11). Moreover, a recent study found that elastography had almost the same diagnostic performance as conventional US with 86% sensitivity, 90% specificity, and 88% accuracy for the differentiation of benign and malignant solid breast masses (12). However, the study had several limitations, i.e., it included palpable breast masses, only one reader analyzed the elastographic images, and the reader was provided with conventional US findings and final pathological information on lesions. Thus, to the best of our knowledge, the diagnostic performance of elastography has not been evaluated for nonpalpable breast masses by blind analysis.
Therefore, we undertook this study to compare the diagnostic performance of conventional US and elastography for the differentiation of benign and malignant nonpalpable breast masses, and to evaluate whether elastography is helpful at reducing the number of benign biopsies, using histological analysis as a reference standard.
This study was conducted with institutional review board (IRB) approval; the requirement for informed consent was waived. Between May 2006 and June 2006, 204 consecutive women who had been scheduled to undergo a US-guided percutaneous needle biopsy based on suspicious imaging findings were examined by US elastography. Of these 204 women; 81 women with palpable masses, 13 with clustered microcalcifications, and 10 with more than one lesion were excluded. A total of 100 nonpalpable breast masses in 100 women (age range, 24-67 years; mean age, 46 years) constituted the study group.
Lesions manifested as clinically occult mammographic lesions in 34 women, a nipple discharge in four, and as an incidental US lesion in 62. Mammograms were available for all women during US examinations. Lesions were observed as a mass in 11 cases, as a mass with microcalcifications in 12, and as a focal asymmetry in 12 cases. One woman with a nipple discharge had a mass with microcalcifications by mammography. No mammographic abnormality was found for 65 (65%) lesions (including 3 women with a nipple discharge). According to the American College of Radiology (ACR) Breast Imaging Reporting and Data system (BI-RADS), the final assessments of the 100 solid breast masses determined before biopsy were as follows: category 3 (probably benign) for 18 masses, category 4a (a low suspicion of malignancy) for 65, category 4b (intermediate suspicion of malignancy) for nine, category 4c (moderate suspicion of malignancy) for two, and category 5 (highly suggestive of malignancy) for six. Biopsy was performed on 18 probably benign lesions due to a request by the patient or referring clinician.
Masses were confirmed by US-guided 14-gauge automated gun biopsy (n = 81) or by 11-gauge vacuum-assisted biopsy (n = 19) within 24 hours of US examinations. Surgical excision was performed for 17 masses because of malignant findings following a previous percutaneous needle biopsy. Of the 100 masses, 17 (17%) were malignant and 83 (83%) were benign. The malignant masses included infiltrating ductal carcinoma (n = 15), infiltrating lobular carcinoma (n = 1), and one ductal carcinoma in situ (DCIS), and the benign lesions were 36 fibroadenomas, 11 papillomas, six adenosis, and 30 fibrocystic changes. Imaging follow-ups were performed on 67 (82%) of 83 benign lesions; median follow-up duration was seven months (range 1-12 months) and lesion stability was confirmed in all. Sixteen lesions without follow-up, were 8 fibroadenomas, one papilloma, one adenosis, and six fibrocystic changes. The histologically determined diameters of lesions were 5-30 mm (mean, 15.2 mm) for the 16 invasive cancers and 30 mm for the one DCIS. Mass sizes determined by US ranged from 5 to 21 mm (mean, 9.8 mm) for benign lesions.
Conventional US and elastographic images were obtained using a EUB-8500 scanner (Hitachi Medical, Tokyo) with a 14-6 MHz linear transducer, by one of three radiologists with 1-5 years of experience of performing breast US and with knowledge of clinical and mammographic findings.
The scanning protocol included transverse and longitudinal real-time imaging of target masses. A split-screen imaging mode was used for conventional US and elastography to obtain identical images. For elastography, the same depths, focus positions, and gain settings were used as for conventional images. A probe was applied to the breast and focused on the target lesion with and without light pressure. The radiologist who performed the real time imaging selected representative transverse and longitudinal images of solid masses obtained by conventional US and elastography. Images were saved in a PACS (picture archiving and communications system) as bitmap files on a hard disc. Image J version 1.37 (National Institutes of Health, Bethesda, MD) was used to separate images so that the reviewers could look at conventional US images and elastographic images individually for later blind review. A region of interest (ROI) was drawn manually to indicate mass margins by the radiologist who performed the real time elastography, and this ROI was superimposed on elastographic images using Image J. Two sets of image files (a conventional US image and elastographic image) were masked and randomized.
Two radiologists that did not perform the US examinations analyzed the conventional US and elastographic images by consensus without knowledge of mammographic or clinical information. Two sets of US images were reviewed with a one-month interval. Readers were given instructions that the malignancy risks of each category determined by US were according to the ACR BI-RADS (13). Category 4 was subclassified into 4a, 4b, and 4c; category 4a included risks from > 2% to < 10%, category 4b risks from > 10% to < 50%, and category 4c risks from > 50% to < 95%.
Personal computer-based software (ACDSee™ Classic; ACD Systems, Miami, FL) and a 21-inch video monitor (2,048 × 2,560 × 8-bit pixels; model DR110; Dataray, Denver, CO) were used in a darkened room. To classify elastographic images, the two radiologists analyzed color patterns both in hypoechoic masses and in surrounding breast tissue. Each image was assigned an elasticity score based on a five-point scale according to the classification proposed by Itoh et al. (12). A score of 1 (E1) indicated even strain for the entire hypoechoic lesion. A score of 2 (E2) indicated strain in most of the hypoechoic lesion, with some strain-free areas. A score of 3 (E3) indicated strain at the periphery of the hypoechoic lesion, and not in its center. A score of 4 (E4) indicated no strain in the entire hypoechoic lesion. A score of 5 (E5) indicated no strain in the entire hypoechoic lesion or in the surrounding area (Fig. 1) (12).
Mean elasticity scores were compared for benign and malignant masses using the student's t-test. Two-tailed pvalues of less than 0.05 were considered statistically significant. The sensitivities, specificities, and positive (PPV) and negative predictive values (NPV) of conventional US and elastographic images were calculated for the differentiation of breast masses using histological findings as a reference. In addition, receiver operating characteristic (ROC) analysis was performed to assess and compare diagnostic performances. To summarize overall performances, areas under the ROC curves (Az) were calculated and compared for the two techniques using MedCalc for Windows, version 9.3.1 (MedCalc Software, Mariakerke, Belgium). The statistical significances between Az values are reported at 95% confidence intervals. Mean differences were regarded as being statistically significant at the 5% level when the corresponding confidence interval did not encompass zero. Statistical analyses other than ROC analysis were performed using SPSS version 10 for Windows (SPSS, Chicago, IL). In terms of management regarding follow-up or a biopsy recommendation, disparities between the two techniques were analyzed.
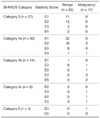
In terms of elasticity scores, the mean ± standard deviation for malignant masses was 3.9 ± 1.1 and for benign masses 1.8 ± s0.8 (p < 0.001) (Figs. 2, 3). For conventional US images, when a cutoff point between category 3 and 4a was used, conventional US had 100% (27 of 27) sensitivity, 33% (27 of 83) specificity, a 23% (17 of 73) PPV, and a 100% NPV (27 of 27). When a cutoff point between category 4a and 4b was used, conventional US had 82% (14 of 17) sensitivity, 89% (74 of 83) specificity, a 61% (14 of 23) PPV, and a 96% (74 of 77) NPV (Table 1). When a cutoff point between E1 and E2 was used, elastography had 100% (17 of 17) sensitivity, 41% (34 of 83) specificity, a 26% (17 of 66) PPV, and a 100% (34 of 34) NPV. When a cutoff point between E2 and E3 was used, elastography had 82% (14 of 17) sensitivity, 84% (70 of 83) specificity, a 52% (14 of 27) PPV, and a 96% (70 of 73) NPV (Table 2). The Az value was 0.901 for conventional US and 0.916 for elastography (95% confidence interval, -0.105 to 0.135), which was not significantly different (p = 0.808) (Fig. 4).
Of the 100 lesions, the management decision as to proceed with a follow-up or a biopsy was discordant for 39 lesions (39%) by conventional US and elastography when a cutoff point between BI-RADS category 3 and 4a and an elasticity score of 1 and 2 was used. Sixteen lesions (16%) were discordant when a cutoff point between BI-RADS category 3 and 4a and an elasticity score of 2 and 3 was used. Forty-five lesions (45%) were discordant when a cutoff point between BI-RADS category 4a and 4b and an elasticity score of 1 and 2 was used. Twenty-four lesions (24%) were discordant when a cutoff point between BI-RADS category 4a and 4b and an elasticity score of 2 and 3 was used. When a cutoff point between BI-RADS category 3 and 4a and an elasticity score of 1 and 2 was used, biopsy was correctly recommended for all malignant lesions. When a cutoff point between BI-RADS category 4a and 4b and an elasticity score of 2 and 3 was used, follow-up was recommended for two malignant lesions rather than biopsy by both conventional US and elastography.
When a cutoff point between BI-RADS category 3 and 4a and an elasticity score of 1 and 2 was used, 23 benign lesions categorized as suspicious by conventional US were correctly recommended as follow-ups by elastography. The 16 benign lesions categorized as suspicious by elastography were correctly recommended as follow-ups by conventional US.
In this study, we evaluated the final assessment categories for conventional US and elastographic classifications of nonpalpable breast masses. It was found that the diagnostic performances of conventional US and US elastography with respect to the differentiation of benign and malignant breast masses were similar. Moreover, areas under ROC curves (Az) were not significantly different for these two methods.
As breast cancers tend to be harder than normal fibroglandular tissues and the breast is readily accessible to compression, palpation has been used to detect and diagnose breast cancers. However, palpation is subjective and lacks sensitivity. Many researchers have developed imaging technologies to measure tissue stiffness objectively. However, no imaging modality can provide a direct measure of intrinsic elastic modulus. The most common elastographic approach involves the imaging of stress and strain. When an object is deformed by an external force (stress), strain is defined as the spatial rate of change of displacement. Strain imaging presents a map of displacement parameters relative to surrounding structure's displacement. Numerous groups have developed semi-quantitative algorithms for strain measurement, and these algorithms have been progressively evolved during the last decade. A few years ago, positioning of patient, data acquisition, and conversion to elastographic images took several hours, and patients were uncomfortable when a motor-driven external compressor was applied. However, today, real time elastographic systems that allow freehand scanning and provide excellent spatial resolution with less noise are integrated into commercial US systems (14). In addition, clinical trials that have focused on the diagnostic performance have been undertaken. Of these early clinical trials, Garra et al. (10) and Hall et al. (11) proposed several diagnostic criteria, which included lesion visualization, relative brightness, margin regularity, and lesion size, after comparing elastograms and B-mode US images. These investigators found that the measured transverse diameters of malignant tumors on elastograms were invariably larger than those measured on conventional US images. Benign tumors show even strain, whereas breast cancers show no strain in lesions or in surrounding areas (12). Moreover, stromal response to breast cancer causes myofibroblasts to produce collagen and extracellular matrix proteins, which increases the stiffnesses of tissue-surrounding tumors and of the tumors themselves (15). Because this strain difference, which is used as a means of producing contrast in elastography, does not cause contrast differences in conventional US images, malignant masses tend to appear larger by elastography, whereas benign masses do not. As this size discrepancy is the key to the differentiation of solid breast masses by elastography, a translucent display of the elasticity image is superimposed on the conventional image, which offers distinct advantages when lesion sizes are compared.
Based on a clinical study, Itoh et al. (12) also proposed an elasticity classification according to the degree and distribution of strain, which concurred with our findings. They reported 87% sensitivity, 90% specificity, and 88% accuracy with a best cutoff point between an elasticity score of 3 and 4, which were similar to values obtained by conventional US (12). However, their study included palpable masses and the investigators evaluated US images with knowledge of physical examination and mammography findings, which may have affected final assessments. As the management of palpable lesions does not depend solely on imaging findings, the performance of elastography for palpable masses has less significance. On the other hand, all lesions included in the present study were nonpalpable masses and 55% of these masses were 4 9 mm in diameter. In addition, we also confirmed that the elasticity classification proposed by Itoh et al. (12) usefully differentiated benign and malignant solid masses, with a diagnostic performance similar to that of BI-RADS categorizations for these nonpalpable, small sized masses. Moreover, the spatial resolution of elastography was sufficient to allow this method to be applied to lesions of less than 1 cm in diameter.
Notably, no malignancy was detected in lesions with an elasticity score of 1 (E1). Of the BI-RADS category 4a lesions, 44% (22 of 50) had E1 and all proved to be benign (Table 1). In terms of management decisions, when a cutoff point between BI-RADS category 3 and 4a and an elasticity score of 1 and 2 was used, 23 benign lesions categorized as suspicious by conventional US were correctly recommended for follow-up by elastography. However, a cutoff point between BI-RADS category 4a and 4b or a cutoff point between an elasticity score of 2 and 3 introduced the possibility of a false negative interpretation. To avoid false negative interpretations and to reduce benign biopsy rates, if a BI-RADS category 4a lesion has even strain in the entire lesion (E1), it might be downgraded to category 3. A further study involving a larger number of cases is necessary to further explore this issue.
Some limitations of the present study should be considered. First, reviewers were not completely unaware of conventional US findings due to the translucency of the elasticity image superimposed on the conventional image, although conventional US and elastographic images were separated and individually reviewed, which probably increased elastographic evaluation performance. Second, imaging follow-ups were performed for 67 (82%) of 83 benign lesions at a median seven months (range 1-12), which was insufficient to confirm lesion stability. Third, one radiologist obtained elastographic images, and only two readers assessed elastographic images by consensus. To obtain appropriate images, it is crucial that light compression be maintained to avoid disruption of the linear association between pressure and strain, and no assessments of image acquisition reproducibility, and intraand inter-observer variabilities in terms of image assessment were performed in the present study. A further evaluation of a larger number of lesions by multiple readers is necessary.
In conclusion, the performances of radiologists with respect to the differentiation of solid breast masses were not significantly different for conventional US and elastography. Moreover, for BI-RADS category 4a lesions, 44% (22 of 50) had an elasticity score of 1 and all lesions were found to be benign. Therefore, when a BI-RADS category 4a lesion has an elasticity score of 1, a biopsy may not be required.
Figures and Tables
Fig. 1
Classification of elasticity scores.
A. Score of 1 (E1) indicated that entire lesion was evenly shaded in green.
B. Score of 2 (E2) indicated that hypoechoic lesion had mosaic pattern of green and blue.
C. Score of 3 (E3) indicated that peripheral portion of lesion was green, and that its central portion was blue.
D. Score of 4 (E4) indicated that entire lesion was blue, but that its surrounding area was not included.
E. Score of 5 (E5) indicated that both entire hypoechoic lesion and its surrounding area were blue.
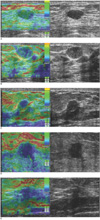
Fig. 2
Transverse conventional US and elastographic images of infiltrating ductal carcinoma in 48-year-old woman.
A. Conventional US showed 1.1 cm ill-defined hypoechoic mass with mild posterior acoustic shadowing.
B. By elastography, entire mass and its surrounding area over margin (white region of interest) were blue, indicating no strain. Final assessment was BI-RADS category 4b by conventional US and score of 5 (E5) by elastography.
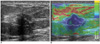
Fig. 3
Transverse conventional US and elastographic images of fibrocystic changes in 65-year-old woman.
A. Conventional US showed 0.5 cm irregular hypoechoic mass.
B. By elastography, mass (white region of interest) appeared green. Final assessment was of BI-RADS category C4a by conventional US and score of 1 (E1) by elastography.
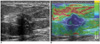
Fig. 4
Receiver operating characteristic curves for conventional US and elastography. Areas under curves were almost same for both conventional US and elastography (0.901 and 0.916, respectively), which was not significantly different (p = 0.808).

References
1. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995. 196:123–134.
2. Rahbar G, Sie AC, Hansen GC, Prince JS, Melany ML, Reynolds HE, et al. Benign versus malignant solid breast masses: US differentiation. Radiology. 1999. 213:889–894.
3. Shin HJ, Kim HH, Kim SM, Kim DB, Lee YR, Kim MJ, et al. Pure and mixed tubular carcinoma of the breast: mammographic and sonographic differential features. Korean J Radiol. 2007. 8:103–110.
4. Park SY, Oh KK, Kim EK, Son EJ, Chung WH. Sonographic findings of breast hamartoma: emphasis on compressibility. Yonsei Med J. 2003. 44:847–854.
5. Steinberg BD, Sullivan DC, Carlson DL. Disparity mapping applied to sonography of the breast: technical note. Radiology. 1998. 207:545–550.
6. Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998. 20:260–274.
7. Wilson LS, Robinson DE. Ultrasonic measurement of small displacements and deformations of tissue. Ultrason Imaging. 1982. 4:71–82.
8. Lerner RM, Huang SR, Parker KJ. Sonoelasticity images derived from ultrasound signals in mechanically vibrated tissues. Ultrasound Med Biol. 1990. 16:231–239.
9. Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991. 13:111–134.
10. Garra BS, Cespedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, et al. Elastography of breast lesions: initial clinical results. Radiology. 1997. 202:79–86.
11. Hall TJ, Zhu Y, Spalding CS. In vivo real-time freehand palpation imaging. Ultrasound Med Biol. 2003. 29:427–435.
12. Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006. 239:341–350.
13. American College of Radiology. Breast imaging reporting and data system (BI-RADS™) ultrasound. 2003. Reston, Va: American College of Radiology.
14. Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003. 23:1657–1671.
15. Insana MF, Pellot-Barakat C, Sridhar M, Lindfors KK. Viscoelastic imaging of breast tumor microenvironment with ultrasound. J Mammary Gland Biol Neoplasia. 2004. 9:393–404.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download