Abstract
Objective
We wanted to evaluate usefulness of uncovered stent in comparison with covered stent for the palliative treatment of malignant colorectal obstruction.
Materials and Methods
Covered (n = 52, type 1 and type 2) and uncovered (n = 22, type 3) stents were placed in 74 patients with malignant colorectal obstruction. Stent insertion was performed for palliative treatment in 37 patients (covered stent: n = 23 and uncovered stent: n = 14). In the palliative group, the data on the success of the procedure, the stent patency and the complications between the two groups (covered versus uncovered stents) were compared.
Results
The technical success rate was 89% (33/37). Symptomatic improvement was achieved in 86% (18/21) of the covered stent group and in 92% (11/12) of the uncovered stent group patients. The period of follow-up ranged from three to 319 days (mean period: 116±85 days). The mean period of stent patency was 157±33 days in the covered stent group and 165±25 days in the uncovered stent group. In the covered stent group, stent migration (n = 11), stent fracture (n = 2) and poor expansion of the stent (n = 2) were noted. In the uncovered stent group, tumor ingrowth into the stents (n = 3) was noted.
Palliative treatment of malignant colorectal obstruction should be considered for the patients suffering with primary disseminated or recurrent cancer. The treatment options depend on the patient's condition, the site of obstruction, the extent of disease and the life expectancy. In many cases and particularly for palliative surgery, creation of colostomy is inevitable, and it may result in increased patient discomfort (1). Various non-surgical treatment procedures such as balloon dilatation, laser photoablation and electrocoagulation have been performed (2-4). However, their effectiveness is limited by the need for repeated treatments that are time-consuming and they increase the patient's discomfort and the medical costs, and these repeated treatments are associated with complications.
Metallic stents have been used for the palliative treatment of malignant obstruction of the biliary and gastrointestinal tracts (5-7). Application of metallic stents for the treatment of acute malignant colonic obstruction was first reported by Dohmoto in 1991, and it has become a promising treatment option (8), although the number of reported cases is only 600 throughout the world (9, 10). According to the reports, stent implantation is the best final palliative treatment for some cases that are in an advanced stage of disease. Yet the patients who undergo palliative treatment develop new colonic obstructions or tumor ingrowth into the stent, and additional complications include stent migration, pain, bleeding and bowel perforation (11-21). To prevent tumor ingrowth and to treat fistulae, a covering has been applied to bare stents, but the stent migration problem still persists, and problems with the newly introduced system still persist (13, 21). To date, there has been no report that has compared the use of covered stents and the use of the uncovered stents in patients with malignant colorectal obstruction.
The purpose of this study was to evaluate the usefulness of uncovered stent in comparison with covered stent for palliative treatment of patients suffering with malignant colorectal obstruction.
From June 1997 to February 2002, 74 patients (35 men and 39 women, age range: 32-85 years, mean age: 60 years) with colorectal obstruction caused by colorectal carcinoma underwent self-expandable metallic stent insertion for decompression of acute colonic obstruction. In 37 patients, the stents were placed for presurgical decompression of obstruction that was caused by primary colonic adenocarcinoma. In another group of 37 patients, colonic stents were placed for palliative relief. The preoperative decompression group of patients was excluded from our study.
The diagnosis of colonic obstruction was initially established on the basis of the patients' clinical history, rectal examinations and the plain radiography. Computed tomography (CT) and barium-enema or water-soluble contrast enema examinations or both were then performed to help confirm the presence of an obstructive mass and to determine the precise location of the lesion. The histopathologic diagnosis was established by analyzing the results of endoscopic biopsy that was performed previously or at the time of the stent insertion. The sites of obstruction were the sigmoid colon in nine patients, the rectosigmoid colon in 24 patients and the descending colon in four patients. The causes of palliation were metastasis (n = 17), seeding (n = 11), local tumor recurrences (n = 8) and rectal cancer with bladder invasion and fistula (n = 1).
We used three types of commercially manufactured stents (CHOO stent; MITech, Seoul, Korea) that were designed for application in the gastrointestinal tract (Fig. 1). The original stent was a flexible covered metallic stent that had three parts: the proximal part, the body (middle portion of the stent) and the distal part. The body of the stent was constructed of 0.4-mm stainless steel wire in a cylindrical zigzag configuration of 15 bends; it was 22 mm in diameter and 10 mm long. The proximal and distal parts were 8 mm wider in diameter than the body of the stent, and the length was 2 cm long; these parts were connected at a right angle to the body by three metallic struts. The proximal and distal parts were constructed of 0.5-mm diameter stainless steel wire with the same mesh. The stent mesh was covered with polyurethane to inhibit growth of the tumor into the stent. There were 3 mm gaps made of only polyurethane that were located between the metallic stent of the body to maintain its longitudinal flexibility. The thickness of the polyurethane coverage at the gap portion was 100-200 µm at the early stage of the study, and it was increased to 300-500 µm during the later period. Two types of flexible covered stents were constructed. Type 1 stents were fully covered with polyurethane. Type 2 stents were not covered in two-thirds of the proximal part so as to prevent their migration.
The type 3 stent was an uncovered stent that was made of 0.17 mm diameter nitinol wires. The wires were knitted in a closed zigzag configuration, joined by plenty of bends, and they were wound cylindrically with looped ends. The stent was from 22 mm to 28 mm in diameter. A gold wire was attached to both ends of the stent to solve the problem of the radiopacity of the nitinol wire. The length of the stent varied from 6 to 16 cm.
The covered stent was mounted on a polytetrafluoroethylene introduction tube that was 6 mm at the outer diameter. The outer diameter of the introducing tube of the uncovered stent was 10.5 Fr. The pusher catheter was made of polyurethane. The delivery system was composed of a guiding tip, a guiding tube, an introduction tube, a pusher catheter and the compressed stent. The patients were divided into three groups: Type 1 stents (n = 10) were used between June 1997 and December 1997, type 2 stents (n = 42) were used between January 1998 and February 2000 and type 3 stents (n = 22) were used between August 2000 and February 2002.
The details of the procedure have been described previously (21). For sedation, 1-2 mg of midazolam (Versed; Roche Laboratories, Nutley, NJ) was administered intravenously before starting the stenting procedure. Under fluoroscopic guidance, a 145-cm-long, 0.038-inch-diameter hydrophilic guide wire (Terumo, Tokyo, Japan) was advanced across the stricture. After an angiographic catheter was advanced above the stricture over the guide wire, the guide wire was removed and a water-soluble contrast material (Telebrix, Guerbet, France) was injected to define the length of the obstruction. The length of obstruction was measured by withdrawing the guide wire from the proximal portion of the obstruction to its distal port. A 0.038-inch-diameter guide wire (Amplatz Superstiff; Meditech/Boston Scientific, Watertown, MA) was then introduced. After an adequate study of the location and the length of the stricture, the delivery system of the stent was coated with lubricating jelly and advanced over the guide wire under fluoroscopic guidance. A stent at least 4 cm longer than the stricture segment was placed so that the stricture was fully covered. The pusher catheter was then held in place while the introducing tube was withdrawn. This maneuver released the stent and allowed it to expand within the stricture. After the deployment of the stent, the delivery system and the guide wire were removed, and a water-soluble contrast enema was performed to assess the position and patency of the stent and to evaluate for any complications that might have occurred during the procedure. In lesions that failed to pass the guide wire with fluoroscopic guidance, we tried to pass the obstructed lesion under combined fluoroscopic and endoscopic guidance.
After the stent placement, a plain radiograph of the abdomen was obtained at 24, 48, and 72 hours, and we evaluated the position of the stent and the relief of the colonic obstruction. After the colonic obstruction was relieved, a barium enema was performed to exclude the possibility of synchronous carcinoma. An additional barium enema study was performed when the patients showed symptoms of colonic obstruction or stent migration. The observation parameters of the follow-up examination included the symptoms, the stent patency and the complications.
Technical success was defined as stent insertion at the lesion site without immediate complications. Clinical success was defined as relief of the bowel obstruction according to the clinical and radiological findings within 24 hours after the stent placement. The period of patency was calculated from the time of stent placement to the recurrence of symptoms of obstruction in the clinically successful cases. The rates of technical success, clinical success and complications were analyzed using the chi-square test, and the Kaplan-Meier method was used to analyze the mean period of patency and the survival time between the covered and the uncovered stents.
The overall technical success rate of stent placement was 89% (33/37). The procedure was performed under fluoroscopic guidance alone in 32 cases; combined guidance was required in five patients. Despite the combined fluoroscopic and endoscopic guidance, we failed to pass the guide wire over the obstructive segment in three cases. In one case, the delivery system could not get over a tortuous rectosigmoid region after the stiff guide wire had crossed the obstruction. Emergency laparotomy operations were performed in both of these two patients, and colostomy or Hartmann's procedure was completed.
In 88% (29/33) of the patients, the clinical manifestations and radiographic findings of the bowel obstruction resolved within 24 hours after stent placement. In patients with resctosigmoid seeding by advanced gastric carcinoma (n = 2), cervical cancer (n = 1) and ovarian cancer (n = 1), their bowel obstruction was relieved two or three days after stent placement.
Complications were noted in 15 patients. The stent migration rate was 33% (11/33) and the duration between stent placement and the migration was 3-72 days (mean: 14 days). Stent migration developed in all the cases (4/4) with type 1 stent and in seven cases with type 2 stent. Stent migration developed within a week in one patient and between one and three weeks in the three patients with the type 1 stent. For the type 2 stent, migration was noted within one week in five patients and after a week in two patients.
Stent migration was not noted in the type 3 stent patients. The migration rate was significantly higher in the covered stent group than in the uncovered stent group of patients (p = 0.009). In three of the cases with migrated stent, the stent was expelled out of the anus. For migration of the type 1 stent, a second stent was inserted to two patients, colostomy was performed to one and no further treatment was given to one patient. For migration of the type 2 stent, a second stent was inserted for two patients, colostomy was performed for two and no further treatment was given to three patients.
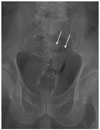
For the type 2 stent, stent fracture was noted in two cases, which occurred 14 days in one and 180 days in the other after stent insertion (Fig. 2). Stent fracture was noted incidentally on the regular follow-up radiography examinations without any specific symptom. Insertion of a second stent was performed in one patient, and surgical operation with colostomy was performed in another patient.
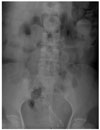
For two cases where the stent did not expand adequately, the patients revealed tumor seeding by cervical cancer and ovarian cancer, respectively (Fig. 3). Insertion of a second stent was performed in one patient, and surgical operation with colostomy was performed in the other patient.
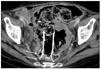
Tumor ingrowth, which was confirmed by CT, developed in three patients with the type 3 stent, but further bowel obstruction was not noted before death (Fig. 4). The tumor ingrowth rate was significantly higher in the uncovered stent group (p = 0.014). In the uncovered stent group, one patient developed symptoms of reobstruction 35 days after the stent placement. The cause of the symptoms was fecal impaction, and the symptoms improved with administering a bowel enema. There was no case of tumor overgrowth in this study.
Minor complication, such as abdominal pain, mild bleeding and tenesmus, improved with conservative treatment.
The period of the follow-up was 3-319 days (mean: 116±85 days). The mean period of patency was 157±33 days with the covered stent and 165±25 days with the uncovered stent: the difference was statistically insignificant (p = 0.481). The mean survival time was 142 days (range: 55-319 days).
Patients with unresectable primary or recurrent malignant colonic obstruction at presentation represent a great medical and ethical dilemma (22, 23). They have a limited life expectancy and they may benefit from a safe, effective and non-surgical alternative treatment to relieve their obstructive symptoms. The primary goal of the non-surgical approach to the treatment of acute colonic obstruction is to eliminate the need of performing urgent surgical treatment on the inadequately prepared colon in an insufficiently stabilized patient.
Treatment of acute colorectal obstruction with using metallic stents is a recent advance that has been mainly used for preoperative decompression (12, 20, 24). In recent years, some authors have reported favorable results for non-surgical relief of acute malignant colonic obstruction by placing an uncovered, self-expandable metallic stent (11-13, 16-19). The technical and clinical success rates of our study are comparable to other series, which varied between 80% and 100% (11-21, 24-28). The site of the lesion influences the technical success rate, with rectosigmoid lesions being easier to treat than transverse colon lesions. Among our cases, technical failure within the descending colon occurred in two cases, within the sigmoid colon in one case and within the rectosigmoid colon in one case.
Generally speaking, the covered stents have the advantage of resisting tumor ingrowth, but they tend to be less stable and more rigid. They also require larger delivery systems; thus, it is more difficult to deploy them at distant locations through a tortuous path of delivery (13, 21). Using a covered stent is favorable because these stents have potential advantages in respect of the longer stent patency, the prevention of tumor ingrowth during palliative treatment and they exclude tumor-induced fistulae (13, 21). Uncovered stents are more flexible, and at least one variety of them can be passed through the working channel of the endoscope. For the purpose of immediate decompression and scheduled surgery, an uncovered stent of a correct length is the optimal choice. However, when used for the long-term palliative treatment of malignant obstruction, they are subject to tumor ingrowth and the resultant obstruction. Rey et al. have reported that early occlusion of uncovered stents occurred in all their eleven patients during 45-91 days of follow-up (14). Because stents made specifically for entral use were not available until 1998, they used stents smaller in the diameter (10-20 mm) than those we are currently using, and that type of stent may be the reason for the early occlusion of the stents. A stent has recently been marketed specifically for use in the colon. According to a recent report on using uncovered stents for palliation of malignant colorectal obstruction, reobstruction due to tumor ingrowing or overgrowing occurred in 7.6 months (range: 4-10 months), and the mean survival time was 5.3 months (16). Another research reported that in patients who had uncovered stents used for palliation of unresectable secondary colorectal carcinoma, the patency of the stent was longer than the patients' survival time (17). Although three cases of tumor ingrowths were observed on CT examinations in our study, there were no intestinal obstructions during the patients' survival in the uncovered stent group. Therefore, coverage did not have a clinically significant influence in the cases treated for palliative relief of bowel obstruction. According to the results of our study, the uncovered stents showed a lower complication rate with no difference in the patency rate in the palliative group. Therefore, we now prefer to use the bare stent because of its comparably high stent patency, the lower migration rate and the stable stent mesh that doesn't fracture.
Complications of colorectal stenting include perforation, migration, pain, tenesmus, bleeding, tumor ingrowth or overgrowth and stent fracture. The complication rate has been reported to range from 14% to 42% (11, 12, 21, 24-26). In various studies, migration has been reported to happen in 4% to 23% of the cases (20, 27). In our study, all the migrations, except one, occurred within 30 days. The established causes of stent migration were the shrinkage of tumors that occurred as a result of adjuvant chemotherapy (12), a weak radial expansive force (14) and a covered stent (21). We think that the principal mechanism of stent migration was associated with the unstable attachment of the stent to the intestinal wall due to the stent coverage and peristaltic movements of the bowel. After we designed a new uncovered nitinol stent, no cases of stent migration were noted.
Stent fracture and inadequate expansion were noted in only a covered stent in our study. This was probably caused by the characteristics of the stent used in this study. In the manufactured covered stents, the stent mesh was not an interconnected one body, but it consisted of segment parts connected by the covering of polyurethane, so the stent could be easily fractured if the covering material was damaged. This could also be caused by the weak radial force in the structure of the stent. More frequent fractured stent struts in the covered stent were considered to be the result of unbalanced mechanical stress due to the covering material and a big stent burden.
Perforation of the bowel is the most dangerous complication because the subsequent fecal peritonitis can be fatal. Colonic perforation following stent deployment is a well recognized problem, and it is more common when balloon dilatation is used to predilate the stricture (9). None of the patients had bowel perforation in this series.
This study has several drawbacks. As the stents were applied differently during a specific period and they were not randomized, this study could not achieve proper comparative analysis, so it has a statistical limitation. A prospective randomized study is needed to overcome this limitation.
In conclusion, favorable technical and clinical results were obtained for palliative treatment with stent insertion for treating malignant colorectal obstruction. Complications developed less frequently with the uncovered stent than with the covered stent, and stent migration especially became less frequent, and the purpose of palliation seems to have been achieved.
Figures and Tables
Fig. 1
Type 1 (A), type 2 (B) and type 3 (C) stents. To prevention migration, two-thirds of the proximal part of the type 2 stent is not covered (arrowheads).

Fig. 2
Stent fracture of the type 2 stent in a 64-year-old patient.
180 days after stent insertion, the stent broke between the
proximal end and the body (arrows).
References
1. Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD. Quality of life in stoma patients. Dis Colon Rectum. 1999. 42:1569–1574.
2. Stone JM, Bloom RJ. Transendoscopic balloon dilatation of complete colonic obstruction. An adjunct in the treatment of colorectal cancer: report of three cases. Dis Colon Rectum. 1989. 32:429–431.
3. Spinelli P, Mancini A, Dal Fante M. Endoscopic treatment of gastrointestinal tumors: indications and results of laser photocoagulation and photodynamic therapy. Semin Surg Oncol. 1995. 11:307–318.
4. Hoekstra HJ, Verschueren RC, Oldhoff J, van der Ploeg E. Palliative and curative electrocoagulation for rectal cancer. Cancer. 1985. 55:210–213.
5. Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992. 340:1488–1492.
6. Bethge N, Sommer A, Vakil N. Palliation of malignant esophageal obstruction due to intrinsic and extrinsic lesions with expandable metal stents. Am J Gastroenterol. 1998. 93:1829–1832.
7. Adam A, Morgan R, Ellul J, Mason RC. A new design of the esophageal Wallstent endoprosthesis resistant to distal migration. AJR Am J Roentgenol. 1998. 170:1477–1481.
8. Dohmoto M. New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endoscopia Digestiva. 1991. 3:1507–1512.
9. Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002. 89:1096–1102.
10. Keymling M. Colorectal stenting. Endoscopy. 2003. 35:234–238.
11. De Gregorio MA, Mainar A, Tejero E, Tobio R, Alfonso E, Pinto I, et al. Acute colorectal obstruction: stent placement for palliative treatment-results of a multicenter study. Radiology. 1998. 209:117–120.
12. Camunez F, Echenagusia A, Simo G, Turegano F, Vazquez J, Barreiro-Meiro I. Malignant colorectal obstruction treated by means of self-expanding metallic stents: effectiveness before surgery and in palliation. Radiology. 2000. 216:492–497.
13. Repici A, Reggio D, De Angelis C, Barletti C, Marchesa P, Musso A, et al. Covered metal stents for management of inoperable malignant colorectal strictures. Gastrointest Endosc. 2000. 52:735–740.
14. Rey JF, Romanczyk T, Greff M. Metal stents for palliation of rectal carcinoma: a preliminary report on 12 patients. Endoscopy. 1995. 27:501–504.
15. Kang SG, Jung GS, Cho SG, Kim JG, Oh JH, Song HY, et al. The efficacy of metallic stent placement in the treatment of colorectal obstruction. Korean J Radiol. 2002. 3:79–86.
16. Hunerbein M, Krause M, Moesta KT, Rau B, Schlag PM. Palliation of malignant rectal obstruction with self-expanding metal stents. Surgery. 2005. 137:42–47.
17. Miyayama S, Matsui O, Kifune K, Yamashiro M, Yamamoto T, Kitagawa K, et al. Malignant colonic obstruction due to extrinsic tumor: palliative treatment with a self-expanding nitinol stent. AJR Am J Roentgenol. 2000. 175:1631–1637.
18. Spinelli P, Mancini A. Use of self-expanding metal stents for palliation of rectosigmoid cancer. Gastrointest Endosc. 2001. 53:203–206.
19. Law WL, Chu KW, Ho JW, Tung HM, Law SY, Chu KM. Self-expanding metallic stent in the treatment of colonic obstruction caused by advanced malignancies. Dis Colon Rectum. 2000. 43:1522–1527.
20. Saida Y, Sumiyama Y, Nagao J, Takase M. Stent endoprosthesis for obstruction colorectal cancers. Dis Colon Rectum. 1996. 39:552–555.
21. Choo IW, Do YS, Suh SW, Chun HK, Choo SW, Park HS, et al. Malignant colorectal obstruction: treatment with a flexible covered stent. Radiology. 1998. 206:415–421.
22. Longo WE, Ballantyne GH, Bilchick AJ, Modlin IM. Advanced rectal cancer. What is the best palliation? Dis Colon Rectum. 1988. 31:842–847.
23. Moran MR, Rothenberger DA, Lahr CJ, Buls JG, Goldberg SM. Palliation for rectal cancer. Resection? Anastomosis? Arch Surg. 1987. 122:640–643.
24. Mainar A, De Gregorio Ariza MA, Tejero E, Tobio R, Alfonso E, Pinto I, et al. Acute colorectal obstruction: treatment with self-expandable metallic stents before scheduled surgery-results of a multicenter study. Radiology. 1999. 210:65–69.
25. Baron TH, Dean PA, Yates MR 3rd, Canon C, Koehler RE. Expandable metal stents for the treatment of colonic obstruction: techniques and outcomes. Gastrointest Endosc. 1998. 47:277–286.
26. Liberman H, Adams DR, Blatchford GJ, Ternent CA, Christensen MA, Thorson AG. Clinical use of the self-expanding metallic stent in the management of colorectal cancer. Am J Surg. 2000. 180:407–412.
27. Canon Cl, Baron TH, Morgan DE, Dean PA, Koehler RE. Treatment of colonic obstruction with expandable metal stents: radiologic features. AJR Am J Roentgenol. 1997. 168:199–205.
28. Dauphine CE, Tan P, Beart RW Jr, Vukasin P, Cohen H, Corman ML. Placement of self-expanding metal stents for acute malignant large-bowel obstruction: a collective review. Ann Surg Oncol. 2002. 9:574–579.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download