Abstract
We report the computed tomographic and angiographic findings in the case of a recently obtained successful clinical outcome after embolization of the hepatic artery in the case of a snakebite causing hemoperitoneum associated with hepatic necrosis and rupture with active bleeding.
Snakebites are a relatively common occurrence worldwide and are estimated to affect greater than 2.5 million humans annually, of whom more than 100,000 will die (1). Imaging manifestations associated with this entity have rarely been described in the scientific literature. We recently obtained a successful clinical outcome after gelatin sponge sheet (Spongostan; Johnson & Johnson, Skipton, UK) embolization of the hepatic artery in the case of a snakebite which caused hemoperitoneum associated with hepatic necrosis and rupture. Until now, this type of complication after a snakebite has not been reported in the literature. We report the computed tomographic and angiographic findings in a case of a snakebite resulting in hemoperitoneum caused by hepatic rupture and necrosis with active bleeding.
An 82-year-old woman was admitted into a rural hospital for treatment of a snakebite on her right index finger which also caused swelling and pain in her right arm. She responded to conservative treatment for three days. After the fourth day, she was referred to our institute due to the sudden development of hypotension, coagulopathy, and anemia. Upon examination, she had pallor, tachycardia, and hypotension. One hour after admission, she complained of abdominal pain and upon further physical examination, abdominal distension with guarding developed. Our study subject had no prior history of hepatitis; however, our laboratory findings showed hemoglobin (7.7 g%), mild leukocytosis, platelet (103,000/mm3), aspartate transaminase (83 IU/L), and alanine transaminase (41 IU/L). In addition, the study subject's prothrombin time (60% and INR: 1.42) and activated prothrombin time (41.8 sec) was prolonged. Moreover, the fibrinogen degradation product level was 160 µg/mL and the D-dimer was positive.
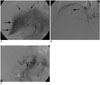
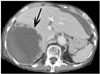
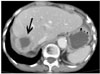
An unenhanced CT revealed a high attenuation of fluid collection in the whole abdominal and pelvic cavity. The study subject's perihepatic hematoma-yielding attenuations were 20-50 HU (Fig. 1A) and a contrast-enhanced CT showed an irregular interface (Fig. 1B) between the hepatic parenchyma and perihepatic hematoma; presumably representing the site of hepatic rupture and multiple active contrast extravasations from the irregular hepatic surface (Fig. 1C). A conventional celiac angiogram (Fig. 2A) showed multiple small contrast extravasations in the peripheral branch of both hepatic arteries. The right inferior phrenic angiogram (Fig. 2B) also showed focal contrast extravasation. The embolization of both hepatic arteries with a small volume of gelatin sponge sheet (Spongostan; Johnson & Johnson, Skipton, UK) until the occlusion of the fourth or fifth ordered branch of the right and left hepatic arteries, and selective coil embolization of the right inferior phrenic artery were performed. The post-embolization angiogram (Fig. 2C) revealed no evidence of contrast extravasation. The general condition of the patient improved gradually, with an increase in serum hemoglobin to 12.3 g% with the coagulation profile corrected to normal levels. A follow-up contrast-enhanced CT (Fig. 3) two weeks after embolization showed a large post-hemorrhagic pseudocyst formation in the necrotic right lobe of the liver and perihepatic space. A follow-up CT (Fig. 4), 16 weeks after embolization, showed a decrease in the size of the post-hemorrhagic pseudocyst after the insertion of a drainage tube and gradual atrophy of the right lobe of the liver.
Globally, venomous snakebites are estimated to affect greater than 2.5 million humans annually, of whom more than 111,000 will die (1). Many potential symptoms may arise in humans as a result of being envenomated by a snake, however; just a few categories are of major clinical significance (2). The potential symptoms include: 1) flaccid paralysis; 2) systemic myolysis; 3) coagulopathy and haemorrhage; 4) renal damage and failure; 5) cardiotoxicity; 6) local tissue injury at the bite site. Snake venom toxins affecting haemostasis have been classified by virtue of their overall effect and include the following: 1) coagulant (the thrombin-like enzymes and prothrombin activating toxins); 2) anticoagulant (toxins activating Protein C etc.); 3) platelet-activating and anti-platelet function (including the disintegrins), a group of RGD-containing proteins); 4) fibrinolytic activators; and 5) haemorrhagins. These effects have been reviewed (2).
In addition to the aforementioned effects, a literature review revealed that complex venomous effects may lead to further complications associated with coagulopathy following a snakebite. The most prominent manifestations of systemic envenoming by viper species include spontaneous bleeding and incoagulable blood and are also two of the most. CT finding of the intramural hematoma in the alimentary tract. Moreover, active contrast extravasation into the peritoneal cavity has been reported in Russel's viper bite (3). Additional reports have included thrombosis of the internal iliac vessels resulting in ischemic colitis with colonic stricture due to disseminated intravascular coagulation from viper toxin (4), ischemic colitis after a viperine snakebite, which resulted in an emergency laparotomy revealing a necrotic ileum and cecum with the occlusion of the superior mesenteric artery (5), and intracranial hemorrhaging and ischemic stroke following various snakebites also has been reported (6).
In our case, the hepatic necrosis in addition to rupture with active bleeding after a snakebite has not been reported in the literature. The other report of nontraumatic hepatic hemorrhage and rupture was a rare complication of HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome. Although the pathogenesis of this condition remains unclear, histopathologic findings in the liver include intravascular fibrin deposits which presumably may lead to hepatic sinusoidal obstruction, intrahepatic vascular congestion, and increased intrahepatic pressure with ensuring hepatic necrosis, intraparenchymal and subcapsular hemorrhage, and eventually capsular rupture. Possible pathogenic mechanisms of hepatic hemorrhaging and rupture for the case of HELLP syndrome includes disseminated intravascular coagulation (7).
The viperine species which inhabit South Korea include Agkistrodon halys, Agkistrodon blomnoffii, and Agkistrodon intermedtas. Agkistrodon is a genus of venomous pit viper and most viper venoms exhibit both coagulant and anticoagulant properties in addition to containing various enzymes. The venom of the Korean viper has a thrombin-like enzyme, arginine ester hydrolase, which is a type of serine protease that can affect fibrinogen to form unstable clots which are not stabilized by factor XIII. Although these clots can be easily destroyed by plasmin because they lack in cross-links, either such clots or disseminated intravascular coagulopathy (DIC) could lead to fibrin deposits in the microcirculation which could lead to platelets and coagulation factors being consumed and a secondary fibrinolysis is initiated, leading to bleeding. In addition, the tissue suffers from ischemia through vessel obstruction. The clinical manifestations of this obstruction are bleeding or thrombosis (8). In our patient, the level of fibrinogen degradation product increased to 160 µg/mL, the D-dimer was positive, and the prothrombin/thrombin time was prolonged, which suggests that DIC occurred. The venom of the Korean viper also contains a lupus anticoagulant-like protein (9). Although the mechanism by which antiphospholipids cause thrombosis is unclear, we speculate that it may be through the inhibition of phospholipid-dependent endogeneous anticoagulants, such as antithrombin III, protein C, thrombomodulin, or prostacyslin. Alternatively, they may affect platelet aggregation or cause complement activation. Another important possible mechanism is the venom itself acting directly upon the hepatic arteries. The venom of vipers which contain a complement-dependent vascular damaging factor called hemorrhagin can act on microvessels and cause severe vasospasms and toxic vasculitis (10). Past studies have suggested that hemorrhagin I from Agkistrodon acutus venom can bind specifically to endothelium and directly damages vessels (10). We believe that the possible mechanisms behind hepatic necrosis and rupture with active bleeding, in our case, was caused by a combination of DIC and direct endothelial injuries as a result of a component in the make-up of the venom itself, such as hemorrhagin. This possible mechanism is similar to hepatic hemorrhaging and rupture characteristic of the HELLP syndrome.
In conclusion, snakebites have complex venomous effects. They may lead to various changes in hemostasis. In our case, snakebite-induced coagulopathy by a Korean viper resulted in hepatic necrosis and rupture with active bleeding. To our knowledge, this is the first published case about CT findings of hepatic necrosis and rupture with active bleeding after a snakebite and should be recognized as a possible complication following snakebites.
Figures and Tables
Fig. 1
Unenhanced axial CT scan (A) shows a hematoma in the perihepatic space (arrow). Enhanced CT scans (B, C) show an irregular interface between the hepatic parenchyma and perihepatic hematoma, which presumably represents the site of hepatic rupture (short arrows) and multiple active contrast extravasations (long arrows).
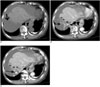
Fig. 2
Hepatic angiogram (A) shows multiple contrast extravasations in the peripheral liver (arrows). A right inferior phrenic angiogram (B) shows the focal contrast extravasation (arrow). A post-embolization angiogram with gelatin sponge (C) shows no evidence of hemorrhage.
References
1. Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1988. 76:515–524.
2. White J. Dart R, editor. Overview of venomous snakes of the world. Medical Toxicology. 2004a. Lippincott: Williams and Wilkins;1543–1559.
3. Rathod K, Sheth R, Chavhan G, Asrani A, Raut A. Hemoperitoneum complicating snake bite: rare CT features. Abdom Imaging. 2003. 28:820–821.
4. Iwakiri R, Fujimoto K, Hirano M, Hisatsugu T, Nojiri I, Sakemi T. Snake-strike-induced ischemic colitis with colonic stricture complicated by disseminated intravascular coagulation. South Med J. 1995. 88:1084–1085.
5. Rosenthal R, Meier J, Koelz A, Müller C, Wegmann W, Vogelbach P. Intestinal ischemia after buchmaster (Lachesis muta) snakebite- a case report. Toxicon. 2002. 40:217–220.
6. Lee BC, Hwang SH, Bae JC, Kwon SB. Brainstem infarction following Korean viper bite. Neurology. 2001. 56:1244–1245.
7. Nunes JO, Turner MA, Fulcher AS. Abdominal imaging feature of HELLP syndrome: A 10-year retrospective review. AJR Am J Roentgenol. 2005. 185:1205–1210.
8. Lee JW, Seu JH, Rhee IK, Jin I, Kawamura Y, Park W. Purification and characterization of brevinase, a heterogeneous two-chain fibrinolytic enzyme from the venom of Korean snake, Agkistrodon blomhoffii brevicaudus. Biochem Biophys Res Commun. 1999. 260:665–670.
9. Li ZY, Wu XW, Yu TF, Lian EC. Purification and characterization of lupus anticoagulant like protein form Agkistrodon halys brevicaudus venom. Thromb Haemost. 1996. 76:791–797.
10. Xu X, Wang Y, Wei C, Zhu X. Study on the action mechanism of hemorrhagin I from Agkidtrodon acutus venom. Adv Exp Med Biol. 1996. 391:361–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download