Abstract
Glomerulonephritis and pulmonary hemorrhage are features of Goodpasture's syndrome. Goodpasture's syndrome accompanied with central nervous system (CNS) vasculitis is extremely rare. Herein, we report a rare case of CNS vasculitis associated with Goodpasture's syndrome in a 34-year-old man, who presented with a seizure and sudden onset of right sided weakness. He also had recurrent hemoptysis of one month's duration. Goodpasture's syndrome is histologically diagnosed by intense linear deposits of IgG along the glomerular basement membrane in both renal and lung tissues.
Goodpasture's syndrome is a rare disease, characterized by rapidly progressive glomerulonephritis, diffuse pulmonary hemorrhage and circulating antiglomerular basement membrane antibody (anti-GBM antibody). Central nervous system (CNS) manifestations in Goodpasture's syndrome are extremely rare, with only a few cases having been reported in the literature (8-10). Therefore, we present our imaging findings of CNS vasculitis associated with Goodpasture's syndrome, together with a review of the relevant literature.
A previously well 34-year-old man presented with generalized tonic-clonic seizure and transient right side weakness. He also had recurrent hemoptysis of one month's duration. For evaluation of the recurrent hemoptysis, a lung CT was performed, which showed multifocal ground-glass opacities in both lungs, consistent with a diffuse alveolar hemorrhage (Fig. 1). Goodpasture's syndrome was suspected; therefore, renal ultrasound was performed. The renal ultrasound demonstrated that his kidney had normal size, shape and echogenicity. To search for the cause of the seizure and transient right side weakness, a brain MRI was performed. The brain MR T2-weighted axial images showed multiple small nodular, high signal intensities in the bilateral cerebellar hemispheres, right frontal and left parietal cortices (Figs. 2A-C). Contrast enhanced T1-weighted axial images showed leptomeningeal enhancement along both parasagittal regions and cerebellar hemispheres. These findings were consistent with multiple small vascular infarcts (Figs. 2D-F). His hemoglobin level was 10.5 g/dL, with a white blood cell count of 6.3×103/mm3, the complement levels were normal. Bacterial cultures obtained during bronchoscopy and virus serology were negative. His echocardiography was negative. We excluded other possible causes, such as septicemia, thrombogenic hematologic disorder, cardiogenic embolism and bacterial endocarditis, and consequently made a provisional diagnosis of CNS vasculitis.
He was anti-GBM antibody negative, with no anti-neutrophil cytoplasmic antibodies (ANCA), double-stranded DNA or anti-smooth muscle antibody. His serum creatine level was normal (1.04 mg/ml, normal range: 0.6-1.2 mg/ml). Urinalysis showed no protein, but a few old form RBCs.
An immunofluorescence study revealed a large amount of total linear and granular IgG deposits in the lung and renal tissues, which suggested Goodpasture's syndrome.
Over a two week period, 14 serial plasmapheresis treatments were given each day, coupled with pulse methylprednisolone therapy. Therefore, the patient made a good neurological recovery.
In 1919, Goodpasture reported the case of an 18-year-old man who developed glomerulonephritis and a pulmonary hemorrhage during a probable influenza epidemic. At autopsy, the patient also displayed systemic vasculitis and hemorrhagic changes in the small intestine, with focal necrosis of the spleen (1). Subsequently, other investigators have described several other similar cases with the same clinical syndrome, which also included rapidly progressive glomerulonephritis and a pulmonary hemorrhage. Most of these cases had no evidence of extrarenal vasculitis; therefore, Goodpasture's syndrome has since been defined as rapidly progressive glomerulonephritis, often accompanied by a pulmonary hemorrhage (2-4).
In 1967, the anti-GBM antibody was identified. The target antigen is a component of the noncollagenous (NC1) domain of the alpha 3 chain of type IV collagen, with the alpha 3 chain being preferentially expressed in the glomerular and pulmonary alveolar basement membranes. After the discovery of the anti-GBM antibody, the classical pathogenesis of Goodpasture's syndrome was modified. Thereafter, the clinical complex of anti-GBM nephritis and a pulmonary hemorrhage has been referred to as Goodpasture's syndrome (5-7).
Extrarenal vasculitis occurs concomitantly in 10 to 30% of Goodpasture's syndrome cases. Particularly, CNS vasculitis associated with Goodpasture's syndrome is extremely rare, with only three cases of the disease having been reported in the literature (8-10). All three of these patients presented with recurrent seizures, with or without hemoptysis. The brain MRI findings of these patients showed diffusely distributed lacunar infarct (8) or multifocal cortical ischemia in the occipital and parietal lobes (9, 10). These findings are similar to those presented by our patient. Although no association between CNS vasculitis and Goodpasture's syndrome has been reported, we assumed that the inflammation of the vessels' wall, due to deposition of the anti-GBM antibody, causes multifocal ischemic lesions and the anti-GBM antibody has a predilection for the small arteries and arterioles. Moreover, Rydel and Rodby suggested the role of an anti-GBM antibody in the development of CNS vasculitis in Goodpasture's syndrome.
Although the gold standard for the diagnosis of CNS vasculitis, including Goodpasture's syndrome, is a biopsy of the leptomeninges and brain, the presumptive diagnosis in most of the previously reported cases was made on the basis of the convincing clinical features, as well as the angiogram or MR imaging findings, which were consistent with CNS vasculitis. In our case, the diagnosis of CNS vasculitis was made on the basis of the clinical features and MR imaging findings. After treatment, the good neurological recovery in our patient supported the diagnosis of CNS vasculitis.
In summary, CNS vasculitis associated with Goodpasture's syndrome is extremely rare. Awareness of the imaging findings, as well as the clinical significance of CNS vasculitis associated with Goodpasture's syndrome, can be helpful in making the correct diagnosis and subsequent management of this rare condition.
Figures and Tables
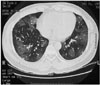 | Fig. 1Lung CT showing multifocal ground-glass opacities in both lungs, suggesting a pulmonary hemorrhage. |
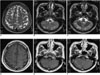 | Fig. 2
A-C. The brain MR T2-weighted axial images showing multifocal small nodular, high signal intensities in the bilateral cerebellar hemispheres, right frontal and left parietal cortices.
D-F. Contrast enhanced T1-weighted axial images showing leptomeningeal enhancement along both parasagittal regions and cerebellar hemispheres.
|
References
1. Goodpasture EW. The significant of certain pulmonary lesions in relation to the etiology of influenza. Am J Med Sci. 1919. 7:863–870.
2. Jayne DR, Marshall PD, Jones SJ, Lockwood CM. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990. 37:965–970.
3. Weber MFA, Andrassy K, Pullig O, Koderisch J, Netzer K. Antineutrophil cytoplasmic antibodies and anti glomerular basement membrane antibodies in Goodpasture's syndrome and in Wegener's granulomatosis. J Am Soc Nephrol. 1992. 2:1227–1234.
4. O'Donoghue DJ, Short CD, Brenchley PE, Lawler W, Ballardie FW. Sequential development of systemic vasculitis with anti-neutrophil cytoplasmic antibodies complicating anti-glomerular basement membrane disease. Clin Nephrol. 1989. 32:251–255.
5. Escolar Castellon JD, Roche PA, Escolar Castellon A, Minana Amda C. Goodpasture's syndrome due to exposure to cigarette smoke. Histol Histopathol. 1991. 6:535–547.
6. Savage CO, Pusey CD, Bowman C, Rees AJ, Lockwood CM. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. Br Med J. 1986. 292:301–304.
7. Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973. 3:74–89.
8. Rydel JJ, Rodby RA. An 18-year-old man with Goodpasture's syndrome and ANCA-negative central nervous system vasculitis. Am J Kidney Dis. 1998. 31:345–349.
9. Garnier P, Deprele C, Pilonchery B, Michel D. [Cerebral angiitis and Goodpasture's syndrome]. Rev Neurol (Paris). 2003. 159:68–70.
10. Nicola G, Anna B, Janet E, Anil G, Nadeem M. Cerebral vasculitis in a teenager with Goodpasture's syndrome. Nephrol Dial Transplant. 2004. 19:3168–3171.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download