Abstract
Hydatid disease (HD) is an endemic illness in many countries, and it poses an important public health problem that's influenced by peoples' socioeconomic status and migration that spreads this disease. Although rare, it may occur in any organ or tissue. The most common site is the liver (59-75%), followed in frequency by lung (27%), kidney (3%), bone (1-4%) and brain (1-2%). Other sites such as the heart, spleen, pancreas and muscles are very rarely affected. Unusual sites for this disease can cause diagnostic problems. This pictorial essay illustrates various radiological findings of HD in the liver, spleen, kidney, pancreas, peritoneal cavity, omentum, adrenal, ovary, lung, mediastinum and retroperitoneum. Familiarity with the imaging findings of HD may be helpful in making an accurate diagnosis and preventing potential complications.
Hydatid disease (HD) is a parasitic disease that's most commonly caused by the larval stage of Echinococcus granulosus. It is still a considerable health problem in the world (1). E. granulosus can reach any organ or tissue in the body where it develops into small hydatid cysts (1-5). The wall of the cyst contains three layers. The outermost layer is the pericyst, the middle layer is the laminated membrane and the innermost layer is the germinal epithelium (endocyst) (1-3, 5). The latter is the only living part of the hydatid cyst and it produces the laminated membrane and infectious scolices that represent the larval stage of the parasite (5). The characteristic imaging findings have been described as calcification of the cyst wall, the presence of daughter cysts and membrane detachment (3). However, the radiological signs are usually non-specific. Serologic tests may be helpful in the diagnosis, but their reliability is not 100% (5). Unusual sites of this disease can frequently cause diagnostic problems and so this can give rise to an increased risk of diagnostic delay and many potentially serious complications. Even a mortality rate of 4% for this disease was reported in the literature (6, 7). In this paper, we describe the radiologic findings of HD in some uncommon sites besides the liver and lung, as based on our experiences.
A pathology-based classification for this disease has been described by Lewall (1). The early lesions (type I hydatid cysts [HCs]) have a non-specific anechoic cystic appearance on ultrasonography (US) (1-4). On CT scans, Type I HC appears as a well-defined, round or oval cystic mass with an attenuation density near that of water (3-30 HU) (Fig. 1). On magnetic resonance imaging (MRI), HC is seen as hypointense on T1-weighted images and as marked hyperintense lesions on T2-weighted images. MRI can displays a low-signal-intensity rim that surrounds the cyst (the "rim sign"), which is more evident on the T2-weighted sequences (2, 8).
For type II HCs, many daughter cysts and/or matrix develop within the parent cyst with or without cyst wall calcification (Fig. 2) (1). Daughter cysts, indicating viability, have a lower attenuation value than that of mother cysts on CT scans (Fig. 2). On MRI, daughter cysts may appear slightly hypointense or isointense relative to the maternal matrix on the T1-weighted images and hyperintense on the T2-weighted images. When present, floating membranes are seen as low signal intensity linear structures within the cyst on both the T1- and T2-weighted images (2, 3, 8).
Type III HCs represent a calcified, non-viable degenerated cyst (Fig. 3) (1). US demonstrates cyst calcification as hyperechoic areas with a strong posterior shadow. Cyst calcification can be seen as round, hyperattenuating areas on CT and as hypointense areas on MRI (2, 3).
Complications of HC include rupture and superinfection of type I and II cysts (1). Rupture occurs in 50%-90% of cases. Three types of rupture can occur: contained, communicating and direct (1). In contained rupture, the endocyst ruptures and becomes detached from the pericyst. In communicating rupture, the cyst ruptures into an anatomical diversion structure like the biliary or bronchial tree (1). Direct rupture occurs when both the endocyst and the pericyst are ruptured with the cystic contents spilling into the pleural and peritoneal cavities or a hollow viscus (Fig. 4) (1). The cyst may become considerably smaller and less spherical both in communicating and direct ruptures (1). MRI may demonstrate disruption in the low-signal intensity rim of the cyst wall and extrusion of the contents through the defect (3, 8).
The liver is the most common site of HC. The cysts may cause pain, discomfort, abdominal swelling or a palpable mass or thrill (5, 6). In the early stages of the disease, the appearance of HCs may be uncharacteristic and mimic that of simple cysts. However, the double-line sign can often be seen on sonography in unruptured HCs (Fig. 5) (3, 4). Simple liver cysts do not demonstrate internal structures (2, 3), although multiple echogenic foci due to hydatid sand may be seen within the lesion by repositioning the patient on sonography (Fig. 6) (5). On MRI, a low-signal-intensity rim can be helpful to differentiate a unilocular HC from a simple liver cyst (2). Multiple unilocular cysts are indistinguishable from polycystic disease (2, 5). When echinococcal cysts become enlarged, they can produce pericystic biliary tract dilatation due to the mass effect (Fig. 7) (2, 5, 9). Decreasing intracystic pressure, endocyst degeneration, host response, trauma and medical treatment can cause separation of the endocyst from the pericyst (2, 3). Complete collapse of the endocyst results in a sonographic water-lily sign when the parasite lies in the most dependent part of the cyst or this produces an irregular, solid echo pattern (Fig. 8) (5). The wall of HC, even without calcification, is typically seen as a high-attenuated structure on unenhanced CT (Fig. 1) (3). The calcification may occur in the cyst wall or internally in the cyst, and this is detected on radiography in 20% to 30% of liver HCs (Fig. 3) (2, 3, 5). Following the formation of HC, many potential local complications may develop such as rupture, infection (Fig. 9), perforation to the biliary tree (up to 90% of HCs) (Fig. 10), and involvement of the portal venous system (Fig. 11), diaphragm and thoracic cavity (0.6%-16% of the cases with hepatic HCs) (2, 3).
The lung is the second most common location of hematogenous HC spread in adults and it is probably the most common site in children (2, 5). Pulmonary hydatid cysts are often asymptomatic, and they are usually found as incidental findings on routine chest radiography, but occasionally symptoms occur due to the pressure effects on adjacent structures (5). The most prominent radiological finding is a dense, round, well-demarcated opacity that can resemble a neoplasm. Calcification (0.7% of cases) and daughter cysts are rarely seen in lung HD (2, 10). When the growth of the cyst produces erosion in the bronchioles, air between the endocyst and pericyst can produce a "crescent or inverse crescent sign" between the cyst wall and the pericyst (Fig. 12). If air continues to enter the cyst cavity, then the "water lily" sign can be seen (an endocyst membrane floating in the most dependent part of the pericyst cavity) (2, 11). The radiologic appearances of infected cysts are similar to those of a lung abscess: a thick-walled cavity with an air-fluid level and surrounding pneumonia (Fig. 13) (5). When HCs are infected, they can cause a solid appearance and give rise to a diagnostic error such as malignant tumor. Other complications of lung HC include rupture and recurrent acute pulmonary embolism (2).
Renal HD is rare (3% of case), usually solitary and located in the cortex (2). These patients may present with a flank mass, dysuria, pyuria, hematuria, persistent fever, renal stones, hypertension or renal colic (10). Hydatiduria can occur after rupture of the cyst into the collecting system (5). Any form of HC can be seen in renal HD (Fig. 14) (2, 3, 10). Mural calcification and daughter cysts often coexistent. These findings are helpful in the differential diagnosis of HCs from a simple renal cyst, necrotic renal cell carcinoma, renal abscess and infected cysts, but it sometimes can be difficult to differentiate HC from necrotic renal cell carcinoma since calcifications may be encountered in both lesions (2, 10).
Peritoneal HC, either primary or secondary, represents an uncommon but significant manifestation of this disease (approximately 13%). It is always secondary to traumatic or surgical rupture of a hepatic, splenic or mesenteric cyst (2, 3, 10). CT is the modality of choice for these patients because it permits imaging of the entire abdomen and pelvis (3). The lesions are generally multiple and any type of HC can arise anywhere in the peritoneal cavity (Fig. 15). Unilocular cysts (type I) should be distinguished from mesenteric cysts or intestinal duplication cysts (2, 6, 10).
Primary splenic involvement is very rare (less than 2%) (Fig. 16). The symptoms are mainly abdominal pain, splenomegaly and fever. Splenic HCs are usually solitary, and their imaging characteristics are similar to those of hepatic HCs (2, 10). Other splenic cystic lesions such as epidermoid cyst, pseudocyst, splenic abscess, hematoma and cystic neoplasm of the spleen should be considered in the differential diagnosis (10).
Primary HC of the pancreas is rare, representing 0.2-2% of all human infestation. It is usually single and located in the head of the pancreas (2, 10). The clinical symptoms depend on the size and location of the cyst within the pancreas. The lesion in the head of pancreas frequently presents with jaundice due to obstruction of the common bile duct. However, the body or tail lesions rarely cause symptoms. The differential diagnosis includes abscess or cystic neoplasms of the pancreas (10). The typical appearance and location of HCs within the pancreas is not established due to their relatively rare occurrence (2). In this paper we present the MRI features of primary HC of the pancreas located in the tail (Fig. 17). We also present the CT findings of a partially calcified HC located in the tail of the pancreas (Fig. 18).
The adrenal gland is an extremely rare location for HC and it is usually involved as a part of systemic echinococcosis. It is usually asymptomatic and these patients usually present with the symptoms that are caused by space-occupying lesions (12). The imaging features depend on the stage of evolution of the disease (Fig. 19). Unilocular HCs should be differentiated from exophytic renal cysts that originate from the upper pole of the kidney (2).
Ovarian involvement is also very rare and generally secondary to peritoneal spread of daughter cysts due to rupture of a liver HC. Ovarian HCs are usually asymptomatic and they can be discovered incidentally or they may cause irritation or compression symptoms. The ovarian lesion may be unilocular (Fig. 20) or contain daughter cysts that can give rise to a multiloculated appearance, and ovarian lesion should be considered in the differential diagnosis of cystic pelvic masses such as cystadenoma or cystadenocarcinoma (2).
Isolated retroperitoneal HCs are also uncommon and they are usually secondary to the involvement of other organs or to previous surgery. Any type of HC can be seen in the retroperitoneum (2, 13). In this paper we present the CT findings of a case of retroperitoneal type II HC that originated from the right psoas muscle (Fig. 21). There was no evidence of any other organ involvement in this case.
Mediastinal HCs are also rare and they can be solitary or multiple lesions. The symptoms and complications of cyst depend on the size, location and involvement of adjacent structures. The imaging appearance can vary from type I to type III (2) (Fig. 23). The HCs in the mediastinum should be differentiated from cysts of a bronchogenic, pleuropericardial, thymic or enteric origin, and from intramural esophageal cysts such as lymphangioma and anterior meningocele (3).
The occurrence of E. granulosus in some locations of the body is very rare. These anatomic locations may cause difficulties in making the differential diagnosis as E. granulosus is usually not suspected in some locations of the body. Imaging modalities such as US, CT and MRI are helpful in diagnosing this disease. Radiologists, surgeons and physicians should always consider HD in differential diagnosis of a cystic lesion, and especially for the cystic leasions encountered in patients who live in or have come from endemic regions and if any of the previously described imaging features (e.g., calcification, daughter cysts and/or intracystic membranes) are seen. Familiarity with the various imaging appearances of HD may prevent diagnostic delay, and so decrease the risk of life-threatening complications.
Figures and Tables
Fig. 1
Type I hydatid cyst of the liver in an 11-year-old girl. Unenhanced CT scan of the upper abdomen shows a large unilocular hydatid cyst (18 HU) with a high-attenuation wall in the subdiaphragmatic portion of the liver (arrow).
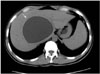
Fig. 2
Type II hydatid cyst in a 36-year-old man. Contrast-enhanced axial CT scan of the upper abdomen demonstrates cystic lesion with peripheral daughter cysts and wall calcification in the left lobe of the liver. Note the daughter cysts have a lower attenuation value than the mother cyst (arrows).
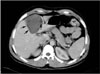
Fig. 3
Type III hydatid cyst in a 28-year-old man. Densely calcified lesion is seen on the right upper quadrant of the abdomen on barium radiography of the stomach.
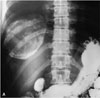
Fig. 4
Direct rupture of hydatid cyst into the peritoneal cavity in a 50-year-old man. Contrast-enhanced axial CT scan of the upper abdomen demonstrates a partially collapsed cystic lesion (black arrow) with an irregular contour growing exophytically from the liver. It has lost its normal spherical shape. Isoattenuating detached membranes (white arrow) appear as serpentine structures within the lesion. A small amount of ascites (white arrowhead) is present.
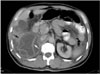
Fig. 5
Type I hydatid cyst of the liver in a 42-year-old woman. At US examination of liver, the cyst wall is seen as double echogenic lines separated by a hypoechoic layer (the double-line sign) (small white arrows). This finding helps to differentiate hydatid cysts from simple cysts, cystic tumors, pseudocysts or metastases.
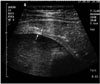
Fig. 6
Type II hydatid cyst is seen on US examination of the liver in a 37-year-old man. Multiple echogenic sand (small white arrows), which is composed of brood capsules and scolices, falls to the dependent portion of the cyst by repositioning the patient. This finding is referred to as the snowstorm sign. Note the early separation of the laminated membrane from the germinal membrane (large white arrow).
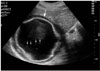
Fig. 7
A. Large type II hydatid cyst of the liver in a 12-year-old girl. Contrast-enhanced transverse CT scan of the upper abdomen demonstrates cystic lesion with a peripheral daughter cyst (short arrow). There is also evidence of pericystic biliary ducts dilatation secondary to the mass effect by a large echinococcal cyst (long arrow).
B. Microscopic section of hydatid cyst in the same patient shows a laminated membrane that is lined inside by the germinal layer (arrowheads). There is also a scolex (arrow) close to the germinal layer. (Hematoxylin & Eosin staining; original magnification × 10)
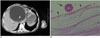
Fig. 8
Type II hydatid cyst mimicking a solid mass in a 25-year-old man. US image demonstrates a mixed echogenic lesion (arrows) of the liver. Note the serpentine structures within the matrix, which represents the collapsed membranes.
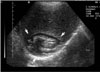
Fig. 9
Infected hydatid cyst of the liver in a 25-year-old woman. Contrast-enhanced CT scan shows a cystic lesion, including air bubbles and collapsed membranes, in the right lobe of the liver (black arrow).
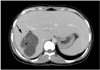
Fig. 10
Rupture of hydatid cyst into the biliary tree in a 54-year-old woman.
A, B. The axial MR image of the upper abdomen using a fast spin-echo T2-weighted sequence shows a hyperintense cystic lesion (short white arrow), including hypointense linear structures representing detached membranes, in the right lobe of liver. There is the hypointense rim (black arrowhead) at the periphery of the lesion. MRI reveals cyst wall defect (black arrow) and the passage of detached membranes through a defect into the extrahepatic bile duct (white arrowhead). Note the biliary dilatation (long white arrows) due to obstruction of the extrahepatic bile duct by hydatid membranes.

Fig. 11
Portal vein involvement of hydatid disease in a 20-year-old woman. The axial (A) contrast enhanced spin-echo T1-weighted sequence (fat-suppression technique) shows a low-signal-intensity lesion in the subdiaphragmatic portion of the right hepatic lobe (white arrow). Thrombosis is identified within the branches of the portal vein (small black arrows). On MR angiography (B), portosystemic venous collaterals (white arrowhead) are seen and the portal vein is not visualized.
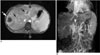
Fig. 12
Multiple lung hydatid cysts in a 30-year-old woman. Axial contrast-enhanced CT of the thorax shows multiple type I hydatid cysts in the lung. There are also ruptured hydatid cysts in the lower lobe of the right lung with the inverse crescent sign (arrow) due to air between the endocyst and pericyst.
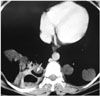
Fig. 13
Infected pulmonary hydatid cyst in a 5-year-old boy. Axial contrast-enhanced CT scan with a mediastinal window setting shows multiloculated low-attenuation lesion with a thick wall in the right lung.
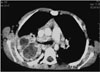
Fig. 14
A 44-year-old-man with type II hydatid cyst of the left kidney. After albendazole therapy, detached germinal membranes are seen within the cyst as low signal intensity linear structures on coronal T2-weighted spin-echo MRI (white arrow).
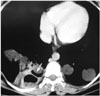
Fig. 15
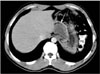
Intraperitoneal hydatid cyst in a 40-year-old woman with a history of liver involvement and previous surgery. The contrast-enhanced axial CT scan revealed multiple cystic lesions (arrows) and ascites (asterisk) in the pelvis.
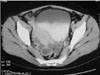
Fig. 16
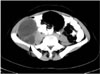
Large type II hydatid cyst with primary splenic involvement in a 50-year-old woman. Contrast-enhanced axial CT scan of the abdomen demonstrates unenhanced, well-defined hypodense lesion with a hydatid matrix and peripheral daughter cysts (arrows) in the upper part of the spleen.
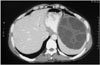
Fig. 17
Type II hydatid cyst with primary pancreas involvement in a 57-year-old woman.
A. Axial MR image of the upper abdomen shows low-signal-intensity lesion with peripheral daughter cysts on the T1-weighted sequence (fat-suppression technique) (arrow). Daughter cysts are seen as lower-signal-intensity lesions compared to the mother cyst.
B. On axial MR image of the upper abdomen, daughter cysts are seen within the mother cyst (short black arrow). The mother cyst and daughter cysts are seen as hyperintense lesions on the fast spin-echo T2-weighted sequence. Also note the hypointense rim at the periphery of the lesion (long white arrow).
C. Microscopic section of hydatid cyst following distal pancreatectomy demonstrates a thick laminated membrane (star) with a thin germinal layer (long arrow). Short arrow shows the pancreas parenchyma.
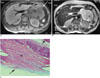
Fig. 18
Partially calcified hydatid cyst of the pancreas in a 27-year-old woman. Contrast material-enhanced CT scan shows a hypodense lesion with dense peripheral calcification (arrow) in the tail of the pancreas.
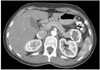
Fig. 19
Type II hydatid cyst of the right adrenal gland in a 47-year-old woman. The axial (A) spin echo T1-weighted MR image demonstrates low-signal-intensity hydatid cyst with peripheral daughter cysts (arrows) in the right adrenal gland. Note that the daughter cysts have lower signal intensity compared to the mother cyst. The sagittal (B) plane spin echo T2-weighted MR image shows linear structures within the mother cyst, which represent detached membranes (long black arrow), and round, nodular lesions within the mother cyst, which represent daughter cysts (short black arrows).
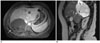
Fig. 20
Incidentally found type I hydatid cyst of the ovary in a 39-year-old woman. The axial (A, B) contrast-enhanced CT scan shows a unilocular low-attenuation lesion (arrows) in the right ovary.
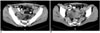
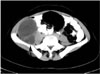
Fig. 21
Type II retroperitoneal hydatid cyst in a 25-year-old woman. The axial contrast-enhanced CT scan through the pelvis demonstrates a hydatid cyst with daughter cysts (arrow) adjacent to the right psoas muscle.
References
1. Lewall DB. Hydatid disease: biology, pathology, imaging and classification. Clin Radiol. 1998. 53:863–874.
2. Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003. 23:475–494.
3. Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000. 20:795–817.
4. Czermak BV, Unsinn KM, Gotwald T, Niehoff AA, Freund MC, Waldenberger P, et al. Echinococcus granulosus revisited: radiologic patterns seen in pediatric and adult patients. AJR Am J Roentgenol. 2001. 177:1051–1056.
5. Beggs I. The radiology of hydatid disease. AJR Am J Roentgenol. 1985. 145:639–648.
6. Erdem LO, Erdem CZ, Karlioguz K, Uner C. Radiologic aspects of abdominal hydatidosis in children: a study of 31 cases in Turkey. Clin Imaging. 2004. 28:196–200.
7. Papageorgiou KI, Kaniorou-Larai M, Mathew RG. An unusual presentation of hydatid cyst within the soft tissues of the back: re-investigation of the undiagnosed lung opacity. Respir Med. 2005. 99:1191–1194.
8. Von Sinner W, te Strake L, Clark D, Sharif H. MR imaging in hydatid disease. AJR Am J Roentgenol. 1991. 157:741–745.
9. Haddad MC, Birjawi GA, Khouzami RA, Khoury NJ, El-Zein YR, Al-Kutoubi AO. Unilocular hepatic echinococcal cysts: sonography and computed tomography findings. Clin Radiol. 2001. 56:746–750.
10. Kiresi DA, Karabacakoglu A, Odev K, Karakose S. Uncommon locations of hydatid cysts. Acta Radiol. 2003. 44:622–636.
11. Koul PA, Koul AN, Wahid A, Mir FA. CT in pulmonary hydatid disease: unusual appearances. Chest. 2000. 118:1645–1647.
12. Escudero MD, Sabater L, Calvete J, Camps B, Labios M, Lledo S. Arterial hypertension due to primary adrenal hydatid cyst. Surgery. 2002. 132:894–895.
13. Dahniya MH, Hanna RM, Ashebu S, Muhtaseb SA, el-Beltagi A, Badr S, et al. The imaging appearances of hydatid disease at some unusual sites. Br J Radiol. 2001. 74:283–289.
14. Canda MS, Guray M, Canda T, Astarcioglu H. The pathology of echinococcosis and the current echinococcosis problem in western turkey (a report of pathologic features in 80 cases). Turk J Med Sci. 2003. 33:369–374.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download