Abstract
Objective
A hepatic mesenchymal hamartoma is an uncommon benign tumor in children and little is known about the spectrum of its radiological features. The purpose of this study is to describe the spectrum of radiological features of a hepatic mesenchymal hamartoma in children.
Materials and Methods
Thirteen children with a pathologically confirmed hepatic mesenchymal hamartoma (M:F = 7:6; mean age, 3 years 2 months) were included in our study. Ultrasonography (US) was performed in nine patients including color and power Doppler US (n = 7). CT scans were performed in all patients. We evaluated the imaging findings of the hepatic mesenchymal hamartomas and the corresponding pathological features.
Results
Each patient had a single tumor (mean diameter: 13 cm [1.8-20 cm]). On CT and/or US, four patients (31%) had a "multiseptated cystic tumor", five patients (38%) had a "mixed solid and cystic tumor", and four patients (31%) had a "solid tumor." The septa of the cystic portion were thin in the multiseptated cystic tumors and irregularly thick in the mixed solid and cystic tumors as seen on US. On a post-contrast CT scan, solid portions or thick septa of the tumors showed heterogeneous enhancement. The amount of hepatocytes was significantly different among the three tumor groups according to the imaging spectrum (p = 0.042).
Amesenchymal hamartoma of the liver is an uncommon benign tumor mostly occurring in children, primarily in the first two years of life. It is thought to be a developmental anomaly of the portal connective tissue rather than a true neoplasm, and is composed of both mesenchymal and epithelial components (1).
Most of the reported cases of a hepatic mesenchymal hamartoma have described a large multiloculated mass with variable septa and cystic spaces (1-5). Rarely, a predominantly solid form of a mesenchymal hamartoma containing a larger amount of hepatocytes has been reported in the literature (6-9).
Although several reports have described the radiological findings of a mesenchymal hamartoma of the liver (3-6), little is known about the spectrum of radiological findings in a series of cases. The aim of this study was to describe a spectrum of the radiological findings of a mesenchymal hamartoma of the liver in children.
We retrospectively reviewed the radiological and clinical findings of 13 patients with a pathologically confirmed mesenchymal hamartoma of the liver. The institutional review board approved the review of the radiological and clinical data for this study and waived the requirement for informed consent. A computed hospital information system was used to identify all patients with a pathologically proved mesenchymal hamartoma during a 15-year period. The pathological diagnosis for a mesenchymal hamartoma was based on overgrowth of the mesenchymal stroma and the proliferation of architecturally abnormal bile ducts with or without a cystic change, accompanied by periductal collaring of the stromal cells (1, 9).
Three pediatric radiologists retrospectively reviewed the radiological features of the hepatic mesenchymal hamartomas by consensus.
CT scans were obtained with various third generation CT scanners at 80-120 mA, 100-120 kVp, and 5- to 10-mm thick sections. CT scans were obtained after an intravenous bolus injection of contrast media and portal venous phase scans of the liver were available for all patients. Pre-contrast CT scans were available for ten patients.
CT images of the tumor were evaluated for location, size, presence of septa within the cystic portion, CT attenuation of the tumor relative to the surrounding hepatic parenchyma (low, iso, or high), pattern of contrast-enhancement (homogeneous or heterogeneous enhancement of the solid portion, and septal enhancement of the cystic portion) and presence of calcification on a pre-contrast CT scan. According to the extent of cystic component on the CT scans, we classified each tumor into one of three types: 1) multiseptated cystic - a purely cystic tumor with multiple thin septa; 2) mixed cystic and solid-a partially cystic tumor with irregular thick septa; 3) solid-a purely solid tumor without a cystic portion. The cystic portion was defined as an area of low attenuation that showed 0-40 Hounsfield units (HU) of CT attenuation value. We classified the septal thickness as follows: thin (< 2 mm in thickness) or thick (> 2 mm in thickness).
Ultrasonographic (US) scans of nine patients were available for review. Among them, seven patients had a color or power Doppler US examination. US images were evaluated for the presence of capsule, internal content of the cystic portion (echogenic debris or fluid-fluid level), septal thickness within the cystic portion, and the echogenicity of the solid portion. Intratumoral vascularity was evaluated by the use of Doppler US.
An experienced hepatobiliary pathologist reviewed the histological sections. The amount of mesenchymal stroma, the presence or absence of duct proliferation, the amount of hepatocytes, and the presence or absence of vascular proliferation was evaluated. The amount of mesenchymal stroma and hepatocytes were evaluated semi-quantitatively: little in < 10% of the mesenchymal hamartoma, moderate in 10-50% of the mesenchymal hamartoma and abundant in > 50% of the mesenchymal hamartoma. The age of the patients, size of the tumor and various histological findings were compared with the imaging features of the hepatic mesenchymal hamartomas among the three CT types using the Kruskall-Wallis test. Statistical analysis was performed with SPSS 11.5 for Windows (SPSS Inc., Chicago, IL). A two-tailed p-value of < 0.05 was considered to be statistically significant.
The subjects were seven boys and six girls, aged from 6 months to 7 years 6 months (mean age: 3 years 2 months). There was no significant difference of age (p = 0.93) among the three CT types of tumors. The patients presented with abdominal distension (n = 8), a palpable mass (n = 3) or an incidentally detected tumor during US (n = 2) for other clinical indications: a patient (case 8) with Peutz-Jeghers syndrome, and a patient (case 13) that had a Kasai operation for biliary atresia at one month of age, respectively. The serum level of alpha-fetoprotein, total and direct bilirubin level, alkaline phosphatase level, and level of transaminases (aspartate transaminase and alanine transaminase) were normal in all except two patients that had an elevated level of alpha-fetoprotein: the patient with biliary atresia (case 13) and a 9-month-old girl (case 5). Hepatitis B surface antigen was negative in all patients. In all patients, the tumors were surgically resected. Clinical features of the patients are summarized in Table 1.
All patients had a solitary hepatic tumor. The tumor occurred in the right lobe of the liver in nine patients and in the left lobe in four patients. Splenomegaly, ascites and retroperitoneal lymphadenopathy were noted in one patient that had biliary atresia.
The longest diameter of the tumors ranged from 1.8 to 20 cm (mean 13.0 cm) and was larger than 10 cm in 11 patients (85%). There was no significant difference in the size of the tumors (p = 0.79) among the three CT types of tumors. The tumor was multiseptated cystic in four patients (31%) (Fig. 1), mixed solid and cystic in five patients (38%) (Figs. 2, 3), and solid in four patients (31%) (Figs. 4, 5).
In four patients with a multiseptated cystic tumor, US (n = 1) showed a well-defined tumor with variable cystic spaces and multiple thin septa in the liver (Fig. 1A). All of these tumors showed fine enhancing septa within the cystic tumor on post-contrast CT (Fig. 1B). Neither calcification nor a solid portion was detected in these patients.
In five patients with a mixed cystic and solid tumor, US (n = 5) showed a well-defined tumor with variable-sized cystic spaces, irregularly thick septa, and a solid portion of variable extent with heterogeneous hyperechogenicity (Figs. 2A, 3A). In three patients, internal debris with fluid-fluid level was seen in the cystic portion of the tumor (Figs. 2A, 2C). On color and power Doppler US (n = 4), linear vascular flow was detected along the thick septa (Fig. 2B) and in the solid portion. On CT, thick septa within the cystic portion (Fig. 2D) and the solid portion of the tumor (Fig. 3C) showed heterogeneous enhancement. A tiny nodular intratumoral calcification was seen in one patient (case 8) (Fig. 3B).
In four patients with a solid tumor, US (n = 3) showed a well-defined, isoechoic tumor (n = 1) or slightly hyperechoic (n =2 ) (Fig. 5A) tumors. Color or power Doppler US (n = 3) showed central (Fig. 5B) or peripheral vascularity in two patients and one patient, respectively. These tumors showed low attenuation on a pre-contrast CT scan in all patients, except for one with a high attenuating lesion. At post-contrast CT, tumors were heterogeneously enhanced in all patients (Figs. 4B, 5C). In the patient that had biliary atresia (case 13), the tumor appeared as a 1.8-cm sized enhancing nodule with a peripheral rim of low attenuation at post-contrast CT (Fig. 5C).
No demonstrable tumor capsule was seen in any patients.
The mesenchymal hamartomas showed multiple variable sized cystic portions within the tumor in their gross morphology (Figs. 1C, 2E, 3D). Each mesenchymal hamartoma with a multiseptated cystic appearance contained various amounts of myxoid or collagenous stroma and showed proliferation of the malformed bile ducts with periductal collaring of the stromal cells (Fig. 1D). All of the four patients with a multiseptated cystic tumor contained a small amount of hepatocytes (< 10%) on a semiquantitative evaluation.
The mesenchymal hamartomas with a mixed solid and cystic appearance also contained various amounts of myxoid or collagenous stroma and showed proliferation of the malformed bile ducts with periductal collaring of the stromal cells (Fig. 2F). Among five patients with a mixed type mesenchymal hamartoma, the amount of hepatocytes was small in three patients and moderate in two patients.
In four patients with a solid mesenchymal hamartoma, a moderate (n = 3) amount or abundant amount (n = 1) of hepatocytes was found in the cords, islands or lobular pattern. The solid tumor also contained various amounts of mesenchymal stroma and proliferation of the small bile duct was seen (Fig. 4D). In one patient with an enhancing nodule with a peripheral rim of low attenuation (case 13), the enhancing portion revealed a large amount hepatocytes histologically with a rim of myxoid stroma. The amount of hepatocytes was significantly different among the multiseptated cystic, mixed solid and cystic, and solid tumors (p = 0.042). The other pathological findings such as the amount of mesenchymal stroma, the presence of a bile duct or vessel proliferation were not statistically significant among the three groups of the different imaging spectrum.
A mesenchymal hamartoma is the second most common benign hepatic tumor in children and accounts for 8% of all hepatic tumors in children (10-14). Histologically, a mesenchymal hamartoma appears as a disordered arrangement of mesenchyme, bile ducts and hepatocytes (1-3, 9, 15). Cords of normal-appearing hepatocytes are separated by zones of loose poorly cellular mesenchyme. The porous nature of a mesenchymal hamartoma permits accumulation of fluid. Cystic degeneration of the mesenchyme with resultant fluid accumulation from obstruction and dilatation of the lymphatics and/or by entrapped bile ducts may lead to enlargement of the tumor (12). The margin between the liver and the tumor is distinct; however, a true capsule is generally not present (2). The level of serum alpha-fetoprotein, which is believed to be secreted by the proliferating hepatocytes within the tumor, can be elevated (13, 14).
A multiseptated cystic appearance, which may be a typical finding of mesenchymal hamartoma, is rarely seen in other hepatic tumors in children. Intratumoral calcification, which can be frequently detected in hepatoblastomas (over 50%) or infantile hemangioendotheliomas (up to 40%) (11), has been reported very rarely for a mesenchymal hamartoma. Chung et al. (8) described the CT findings of a mesenchymal hamartoma containing central and peripheral calcifications in a 57-year-old woman. Konez et al. (15) also described the CT findings of the multiseptated cystic form of a mesenchymal hamartoma with a septal calcification in a 10-year-old boy. In this series, a tiny calcification within the tumor was seen only in one patient.
Although a mesenchymal hamartoma present most frequently as a multiseptated cystic or mixed solid and cystic tumor (1-5), it can rarely occur as a solid tumor (6-9). In this study, according to the extent of the cystic component on CT scans, we classified each tumor into one of three types for evaluating the imaging spectrum of this tumor. In this study, the tumor was totally solid in 31% of the patients, and one case of a solid mesenchymal hamartoma (case 11) showed features of a so-called "mixed hamartoma." The mixed hamartoma is a rare hamartomatous lesion composed of all cellular components of the normal liver, including a larger amount of hepatocytes, ductal proliferation, and hepatocellular-ductal transition. The mixed hamartoma and mesenchymal hamartoma may be in one disease spectrum, since there is some overlapping in the histological features of both hamartomas (16, 17). In children with a solid mesenchymal hamartoma, radiological differential diagnosis should include various hepatic tumors such as a hepatoblastoma, a hepatocellular carcinoma, an infantile hemangioendothelioma, a hepatic adenoma, or a focal nodular hyperplasia. Heterogeneous enhancement and absence of a tumor capsule in a mesenchymal hamartoma can be helpful in the differential diagnosis from other tumors.
As in one of our patients that had biliary atresia and a solid mesenchymal hamartoma (case 13), Lack (18) described a mesenchymal hamartoma in a 5-month-old girl with biliary atresia, which was found incidentally during an exploratory laparotomy for obstructive jaundice. The cystic component of this tumor was either very small or undetectable. Lack (18) showed an interest in this case because one of the theories for development of a mesenchymal hamartoma is biliary obstruction (19).
In conclusion, a mesenchymal hamartoma of the liver in children can show a wide spectrum of radiological features from a multiseptated cystic tumor to a mixed solid and cystic tumor, and even a solid tumor.
Figures and Tables
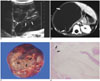 | Fig. 1A mesenchymal hamartoma in a 2-year-8-month-old boy (case 2).
A. US shows a large, multiseptated cystic tumor in the right lobe of the liver. The septa of the tumor (arrows) are very thin and regular in thickness.
B. A post-contrast CT scan shows a large cystic tumor with fine enhancing septa (arrows) in the liver. There is no solid portion or calcification within the tumor.
C. A photograph of the gross specimen shows a huge tumor with a marked ballooning appearance and multiple septa (arrows).
D. A photomicrograph shows variable sized cystic spaces with myxoid mesenchymal stroma and proliferation of the bile duct (arrows) along the septa. (Hematoxylin & Eosin staining, × 40)
|
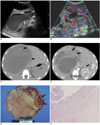 | Fig. 2A mesenchymal hamartoma in a 7-year-2-month-old boy (case 7).
A. US shows a huge, mixed solid and cystic tumor. The echogenic materials and fluid-fluid levels (arrows) are noted in the cystic portions of the tumor. The solid portion of the tumor is hyperechoic (*).
B. Color Doppler US shows vascularity along the thick septa and solid portion of the tumor.
C. A pre-contrast CT scan shows a low attenuating tumor in the right lobe of the liver. Note the fluid-fluid levels due to an intracystic hemorrhage within some of the cystic areas (arrows).
D. On a post-contrast CT scan, the solid portion of the tumor shows heterogeneous enhancement (arrows).
E, F. A photograph of a cut section of the specimen shows a large tumor with a central solid portion and a peripheral cystic portion (arrows, E). The solid portion contains a large amount of myxoid mesenchymal stroma and proliferation of the bile duct, as seen on the photomicrograph (F, Hematoxylin & Eosin staining, × 40).
|
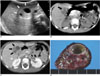 | Fig. 3A mesenchymal hamartoma in a 5-year-4-month-old girl (case 8).
A. US shows a 3-cm-sized solid and cystic tumor (arrows) in the left lobe of the liver.
B. A pre-contrast CT scan shows a tiny nodular calcification (arrow) at the peripheral portion of the tumor.
C. A post-contrast CT scan shows a heterogeneously enhancing tumor (arrows) with focal cystic portions.
D. A photograph of a cut section of the specimen shows variable sized cystic portions within the tumor.
|
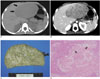 | Fig. 4A mesenchymal hamartoma in a 2-year-5-month-old girl (case 10).
A. A pre-contrast CT scan shows a large, solid tumor (arrows) in the left lobe of the liver. The tumor shows low attenuation compared with the surrounding liver parenchyma on a pre-contrast CT.
B. On a post-contrast CT scan, a heterogeneously enhancing tumor is seen in the left lobe of the liver.
C. A photograph of the specimen shows a solid tumor with a marbling appearance.
D. A photomicrograph shows large amount of myxoid mesenchymal stroma and proliferation of the bile duct (arrows). Hepatocytes are visible toward the left margin of the section (arrows, Hematoxylin & Eosin staining, × 40).
|
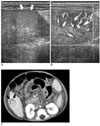 | Fig. 5A mesenchymal hamartoma in a 12-month-old girl that had a Kasai operation for biliary atresia (case 13).
A. US shows a well-defined hyperechoic nodule (arrows) in the tip of the right lobe of the liver.
B. Power Doppler US shows multiple intratumoral vascularities.
C. On a post-contrast CT scan, a well-defined tumor is seen in the right lobe of the liver (arrows). The tumor shows a lobulated central portion with a peripheral rim of low attenuation.
Histologically, the iso-attenuating portion contained a large amount of hepatocytes and a low attenuating rim of the peripheral portion revealed myxoid stroma. Ascites is seen.
|
Table 1
Summary of the Clinical and Radiological Features of Children with a Mesenchymal Hamartoma of the Liver

Note.-Distension = abdominal distension, Detected at US = incidentally detected at US, Septa = septal enhancement of cystic tumor, Heterogeneous-Septa = heterogeneous enhancement of the solid portion and septa , Heterogeneous = heterogeneous enhancement of a solid tumor, N/A = not available, ND = Doppler US examination was not done, † = focal calcification on precontrast CT, * = elevated alpha fetoprotein.
References
1. Dehner LP, Ewing SL, Sumner HW. Infantile mesenchymal hamartoma of the liver. histologic and ultrastructural observations. Arch Pathol. 1975. 99:379–382.
2. Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg. 2005. 40:1681–1690.
3. Ros PR, Goodman ZD, Ishak KG, Dachman AH, Olmsted WW, Hartman DS, et al. Mesenchymal hamartoma of the liver: radiologic-pathologic correlation. Radiology. 1986. 158:619–624.
4. Federici S, Galli G, Sciutti R, Cuoghi D. Cystic mesenchymal hamartoma of the liver. Pediatr Radiol. 1992. 22:307–308.
5. Stanley P, Hall TR, Woolley MM, Diament MJ, Gilsanz V, Miller JH. Mesenchymal hamartomas of the liver in childhood: sonographic and CT findings. AJR Am J Roentgenol. 1986. 147:1035–1039.
6. Cetin M, Demirpolat G, Elmas N, Yüce G, Cetingül N, Balik E. Stromal predominant type mesenchymal hamartoma of liver: CT and MR features. Comput Med Imaging Graph. 2002. 26:167–169.
7. Wada M, Ohashi E, Jin H, Nishikawa M, Shintani S, Yamashita M, et al. Mesenchymal hamartoma of the liver: report of an adult case and review of the literature. Intern Med. 1992. 31:1370–1375.
8. Chung JH, Cho KJ, Choi DW, Lee BH, Chi JG. Solid mesenchymal hamartoma of the liver in adult. J Korean Med Sci. 1999. 14:335–337.
9. Cook JR, Pfeifer JD, Dehner LP. Mesenchymal hamartoma of the liver in the adult: association with distinct clinical features and histological changes. Hum Pathol. 2002. 33:893–898.
10. Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956. 91:168–186.
11. Helmberger TK, Ros PR, Mergo PJ, Tomczak R, Reiser MF. Pediatric liver neoplasms: a radiologic-pathologic correlation. Eur Radiol. 1999. 9:1339–1347.
12. Stocker JT, Ishak KG. Mesenchymal hamartoma of the liver: report of 30 cases and review of the literature. Pediatr Pathol. 1983. 1:245–267.
13. Alwaidh MH, Woodhall CR, Carty HT. Mesenchymal hamartoma of the liver: a case report. Pediatr Radiol. 1997. 27:247–249.
14. Ito H, Kishikawa T, Toda T, Arai M, Muro H. Hepatic mesenchymal hamartoma of an infant. J Pediatr Surg. 1984. 19:315–317.
15. Konez O, Goyal M, Vyas PK, Boinapally SB. Mesenchymal hamartoma of the liver. Comput Med Imaging Graph. 2001. 25:61–65.
16. Chang HJ, Jin SY, Park C, Park YN, Jang JJ, Park CK, et al. Mesenchymal hamartoma of the liver: comparison of clinicopathologic features between cystic and solid forms. J Korean Med Sci. 2006. 21:63–68.
17. Rhodes RH, Marchildon MB, Luebke DC, Edmondson HA, Mikity VG. A mixed hamartoma of the liver: light and electron microscopy. Hum Pathol. 1978. 9:211–221.
18. Lack EE. Mesenchymal hamartoma of the liver. A clinical and pathologic study of nine cases. Am J Pediatr Hematol Oncol. 1986. 8:91–98.
19. Okeda R. Mesenchymal hamartoma of the liver. An autopsy case with serial sections and some comments on its pathogenesis. Acta Pathol Jpn. 1976. 26:229–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download