Abstract
Aspergillosis is a rare cause of spondylitis. Moreover, early diagnosis by MR imaging and adequate treatment can prevent the serious complications of fungal infection. To our knowledge, the MR findings of multilevel aspergillus spondylitis in the cervico-thoraco-lumbar spine have not been previously described. Here, we report the MR findings of aspergillus spondylitis involving the cervical, thoracic, and lumbar spine in a liver transplant recipient.
Aspergillosis is a rare cause of spondylitis, and early diagnosis by MR imaging and adequate treatment are essential for a good outcome (1-3). Although the MR findings of bacterial spondylitis have been fully described (4, 5), the findings of aspergillus spondylitis have been rarely described, and to the best of our knowledge multilevel involvement of cervico-thoraco-lumbar spine has not been previously reported. Here, we report the MR imaging findings of aspergillus spondylitis involving the cervico-thoraco-lumbar spine in a liver transplant recipient.
A 46-year-old man underwent liver transplantation due to hepatitis B virus cirrhosis in March 2005, and subsequently was treated using routine immunosuppression therapy. However, his early postoperative course was complicated by pulmonary aspergillosis. About 10 weeks after liver transplantation a left lower lobe wedge resection and pathology showed aspergilloma, and about three weeks after this thoracic surgery the patient complained of back pain. Plain radiography of the lumbar spine demonstrated no abnormality, and although his back pain was treated conservatively, the patient complained of progressive back pain.
MR imaging of the lumbar spine revealed band-like or diffuse hypointense signals in vertebral bodies L2 to L5 on T1-weighted images (Fig. 1A), which were isointense to slightly hyperintense on T2-weighted images (Fig. 1B). In detail, T1-weighted images showed some hypointense signals with preservation of intranuclear clefts in the L2-3 and L4-5 discs, but disc hyperintensity was absent and intranuclear cleft loss was visualized in the L3-4 disc. In addition, endplate irregularities were apparent in the involved spine. Disc space narrowing was observed at L3-4 and L4-5, and band-like or diffuse enhancement was observed in involved vertebral bodies with an epidural abscess (Fig. 1C). A paraspinal abnormal signal was relatively well-defined (Fig. 1D). The MR based diagnosis was of tuberculous spondylitis rather than pyogenic spondylitis. At surgery, infected granulation tissue and disc material with pus were found at the L3-4 and L4-5 levels, and osteolysis was observed at the inferior endplate of L3, the superior and inferior endplates of L4, and the superior endplate of L5. After curettage and debridement of these lesions, an iliac crest bone block was inserted. Histologically, acute inflammation and necrosis with fungal hyphae suggested aspergillus infection. Histopathologically periodic acid-Schiff and Gomori methenamine silver staining revealed aspergillus infection.
About three months after he underwent lumbar spine MR imaging, he was readmitted for posterior neck pain. MR images of the cervical and thoracic spine showed changes in bone marrow and endplates at the C4-5 and T2-4 levels, the latter of which were similar to the previous findings of lumbar involvement (Figs. 1E-G). Increased disc signal and loss of intranuclear cleft were observed in C4 5 and T2-4 discs. Endplate irregularities and disc space narrowing were observed in the involved spine. During surgery on the cervical spine, infected granulation tissue and disc material were found at the C4-5 level with osteolysis at the inferior endplate of C4 and the superior end plate of C5. Debridement and bone grafting were performed, and pathologic reports disclosed acute inflammation with necrosis and osteomyelitis. Histopathological periodic acid-Schiff and Gomori methenamine silver staining findings revealed aspergillus infection (Figs. 1H, I).
Invasive aspergillosis is a life threatening fungal infection that is associated with a high mortality rate despite treatment. Symptoms and signs are nonspecific. In previous reports times between symptom onset and a definite diagnosis were of the order of months (4-7), and in our patient, symptoms persisted for more than two months before pathologic diagnosis. Ordinary laboratory findings are of little help in the diagnosis of aspergillosis (6).
According to a previous report by Williams et al. (8), the absence of disc hyperintensity and intranuclear cleft preservation on T2-weighted images are suggestive of nonpyogenic spondylitis. In our case, decreased disc signal intensity was observed in three of six affected discs and intranuclear clefts were preserved in two of six affected discs. The minimal hyperintensity or isointensity of vertebrae on T2-weighted images observed in our case is consistent with the findings of previous reports (8, 9) on Candida or Aspergillus spondylitis.
Aspergillus spondylitis is often confused with tuberculous spondylitis (3, 10). In a previous case report (3), MR showed involvements of three consecutive vertebral bodies and a well-defined paraspinal mass, and as a result, tuberculous spondylitis was initially considered. Finally, the patient was pathologically confirmed as having Aspergillus spondylitis. Based on our experiences, the following MR findings favor tuberculous spondylitis rather than pyogenic spondylitis; a well-defined paraspinal abnormal signal rather than an ill-defined paraspinal abnormal signal, a thin and smooth abscess wall rather than a thick and irregular abscess wall, the presence of a paraspinal or an intraosseous abscess, subligamentous spread over at least three vertebral levels, multiple vertebral body involvement, and thoracic spine involvement (5). In the present case, four consecutive vertebral bodies were involved and an abnormal paraspinal signal was relatively well-defined on the initial lumbar spine MRI, which indicated tuberculous spondylitis rather than pyogenic spondylitis. However, no paraspinal or intraosseous abscess was evident despite the extensive involvements of four consecutive vertebral bodies and an abnormal paraspinal signal. Because based on our experiences, about 95% (15/20) of tuberculous spondylitis cases have a concomitant paraspinal or intraosseous abscess (5), the absence of a paraspinal or intraosseous abscess appears to be unusual in tuberculous spondylitis. However, as South Korea is still an endemic area for tuberculosis, and initial diagnosis of tuberculous spondylitis was made despite some inconsistent MR findings. In retrospect, we should have considered a fungal infection, such as, Candida or aspergillus spondylitis in this immunocompromised patient.
In conclusion, aspergillus spondylitis should be considered in the differential diagnosis of immunocompromised patients with MR findings resembling those of tuberculous spondylitis.
Figures and Tables
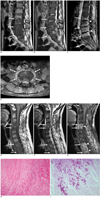
Fig. 1
A 46-year-old man with aspergillus spondylitis.
A, B. MR image of the lumbar spine showing band-like or diffuse hypointense signals (arrows) in vertebral bodies L2-L5 on T1-weighted images (A), whereas these lesions were isointense to slightly hyperintense (arrows) on T2-weighted images (B). Some hypointense signals with intranuclear cleft preservation were evident in L2-3 and L4-5 discs. However, an absence of disc hyperintensity and intranuclear cleft loss were observed in the L3-4 disc. Endplate irregularities were evident in the involved spine, and disc space narrowing was observed in L3-4 and L4-5.
C. Band-like or diffuse enhancement (arrows) was observed in involved vertebral bodies with epidural abscesses (arrowheads) on sagittal fat-suppressed contrast-enhanced T1-weighted image.
D. Axial fat-suppressed contrast-enhanced T1-weighted image showing relatively well-defined paraspinal abnormal enhancement (arrows).
E, F. MR image of cervical spine showing diffuse hypointense signals (arrows) in C4-5 and T2-4 vertebral bodies on T1-weighted images (E). The lesions were isointense to slightly hyperintense (arrows) on T2-weighted images (F). Increased disc signal and loss of intranuclear cleft were observed in C4-5 and T2-4 discs. Endplate irregularities and disc space narrowing were seen in involved spine.
G. Diffuse enhancement was seen in involved vertebral bodies with paraspinal (arrows) and epidural masses (arrowheads) on sagittal fat-suppressed contrast-enhanced T1-weighted images.
H. Pathological examination of an intervertebral disc revealed acute inflammation, necrosis and portions of the tissue being invaded by septate hyphae (Hematoxylin & Eosin staining, ×200).
I. Branching septate hyphae were uniform in width and disposed mainly at acute angles (diastase periodic acid Schiff, ×400).
References
1. Morgenlander JC, Rossitch E Jr, Rawlings CE 3rd. Aspergillus disc space infection: case report and review of the literature. Neurosurgery. 1989. 25:126–129.
2. Park KU, Lee HS, Kim CJ, Kim EC. Fungal discitis due to Aspergillus terreus in a patient with acute lymphoblastic leukemia. J Korean Med Sci. 2000. 15:704–707.
3. Park SB, Kang MJ, Whang EA, Han SY, Kim HC. A case of fungal sepsis due to aspergillus spondylitis followed by cytomegalovirus infection in a renal transplant recipient. Transplant Proc. 2004. 36:2154–2155.
4. Dagirmanjian A, Schils J, McHenry M, Modic MT. MR imaging of vertebral osteomyelitis revisited. AJR Am J Roentgenol. 1996. 167:1539–1543.
5. Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 2004. 182:1405–1410.
6. Chi CY, Fung CP, Liu CY. Aspergillus flavus epidural abscess and osteomyelitis in a diabetic patient. J Microbiol Immunol Infect. 2003. 36:145–148.
7. Stratov I, Korman TM, Johnson PD. Management of Aspergillus osteomyelitis: report of failure of liposomal amphotericin B and response to voriconazole in an immunocompetent host and literature review. Eur J Clin Microbiol Infect Dis. 2003. 22:277–283.
8. Williams RL, Fukui MB, Meltzer CC, Swarnkar A, Johnson DW, Welch W. Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol. 1999. 20:381–385.
9. Munk PL, Lee MJ, Poon PY, O'Connell JX, Coupland DB, Janzen DL, et al. Candida osteomyelitis and disc space infection of the lumbar spine. Skeletal Radiol. 1997. 26:42–46.
10. van Ooij A, Beckers JM, Herpers MJ, Walenkamp GH. Surgical treatment of aspergillus spondylodiscitis. Eur Spine J. 2000. 9:75–79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download