Abstract
Objective
We wanted to evaluate the role of the arterial phase (AP) together with the portal venous phase (PP) scans in the diagnosis of Fitz-Hugh-Curtis syndrome (FHCS) with using computed tomography (CT).
Materials and Methods
Twenty-five patients with FHCS and 25 women presenting with non-specifically diagnosed acute abdominal pain and who underwent biphasic CT examinations were evaluated. The AP scan included the upper abdomen, and the PP scan included the whole abdomen. Two radiologists blindly and retrospectively reviewed the PP scans first and then they reviewed the AP plus PP scans. The diagnostic accuracy of FHCS on each image set was compared for each reader by analyzing the area under the receiver operating characteristic curve (Az). Weighted kappa (wk) statistics were used to measure the interobserver agreement for the presence of CT signs of the pelvic inflammatory disease (PID) on the PP images and FHCS as the diagnosis based on the increased perihepatic enhancement on both sets of images.
Results
The individual diagnostic accuracy of FHCS was higher on the biphasic images (Az = 0.905 and 0.942 for reader 1 and 2, respectively) than on the PP images alone (Az = 0.806 and 0.706, respectively). The interobserver agreement for the presence of PID on the PP images was moderate (wk = 0.530). The interobserver agreement for FHCS as the diagnosis was moderate on only the PP images (wk = 0.413), but it was substantial on the biphasic images (wk = 0.719).
Fitz-Hugh-Curtis syndrome (FHCS) is characterized by inflammation of the liver capsule associated with a genital tract infection (1). Clinically, the diagnosis of FHCS can be difficult because it may mimic many other diseases, including acute cholecystitis, pneumonia, pulmonary embolism, renal colic and perforated peptic ulcer (1). Among them, acute cholecystitis is the most common mimicking disease, especially in cases when right upper quadrant (RUQ) pain is more pronounced. The diagnosis is even more difficult when pelvic symptoms are absent, or when perihepatitis presents long before or after the symptoms of the pelvic inflammatory disease (PID) (1, 2). The incidence of FHCS is variable but not uncommon, and it can be recognized clinically or laparoscopically in 3-37% of patients with PID (1, 3). The incidence can be higher in adolescents (4). In spite of our knowledge about clinical prevalence of this disease, there not so many reports on its imaging features. On ultrasonography, widening and fluid collections can be seen in the right subphrenic area, which is the most common finding associated with this disease, while there is depiction of violin-string appearance in some cases (5-9). On computed tomography (CT), the increased enhancement along the hepatic surface has been described as a finding that can suggest the diagnosis of FHCS (10, 11). However, some atypical findings on the CT scan have also been reported (12-15).
Nishie et al. (10) have shown that the increased perihepatic enhancement can be a more common finding on the early phase scans than on the late phase scans. However, in their study, the 'early' phase scans were taken with a scan delay of 45-60 sec, which might contribute to inclusion of the portal or hepatic venous phase images. In our institution, biphasic scan, including the arterial phase (AP) scan of the upper abdomen and the portal venous phase (PP) scan of the entire abdomen and pelvis, has been adopted as a routine examination protocol for patients with acute RUQ pain. By using this technique we have found that the CT diagnosis of the FHCS can be considerably improved, probably because the depiction of the perihepatic enhancement is facilitated by the inclusion of the arterial phase scan. Therefore, in this study, we compared the diagnostic accuracy of FHCS between the AP plus the PP scan and the PP scan alone, to determine the role of the AP scan for a more precise diagnosis of the FHCS.
One radiologist retrospectively reviewed the radiology and hospital information databases at our institution from February 2004 to February 2005 to identify all patients with the diagnosis of FHCS. A total of 50 female patients were retrieved. Among them, 25 patients were excluded from this study for the following reasons: monophasic contrast enhanced CT was performed (n = 12), the medical records of the patients were not available (n = 6), and the final clinical diagnosis of PID was made without documentation of FHCS (n = 7). Therefore, 25 females (mean age, 30 years; age range, 18-49 years) who underwent the biphasic contrast-enhanced spiral CT examination and who were finally diagnosed as FHCS patients with PID were included in this study. The diagnostic criteria of FHCS were as follows: the presence of RUQ pain, clinically diagnosed PID, the hybrid capture test for Chlamydia trachomatis, leukocytosis or tests for elevation of C-reactive protein response to antibiotics (10). All the patients had RUQ pain. Leukocytosis or elevation of C-reactive protein was noted in 24 patients. The hybrid capture tests of the vaginal swab revealed chlamydia infection in 20 patients. All the patients had signs or symptoms of PID and they all responded well to the antibiotic treatment.
Age-matched control patients were selected among the female patients who presented with acute abdominal pain. They were either clinically diagnosed as PID in the absence of RUQ pain or they could have other equivocal causes of abdominal pain without clinical evidence of PID. Patients with a distinct diagnosis or radiological features of the pain were excluded; hence, patients with acute appendicitis, acute cholecystitis, acute pyelonephritis, ureteral stone and intestinal obstruction were not included. Finally, 25 females (mean age, 30 years; age range, 16-47 years) who underwent the biphasic contrast-enhanced spiral CT examination were selected as the control group. All of the patients in the control group showed recession of symptoms after the antibiotic therapy.
The period from the onset of the abdominal pain to the performance of the CT scans was 2±0.72 days in the patient group and 1±0.84 days in the control group. This study was approved by our institutional review board, and there was no requirement to receive informed consents.
The biphasic CT examinations were performed with a Light Speed Plus 4 multidetector-row CT (MDCT) scanner (GE Medical Systems, Milwaukee, WI) (n = 35) or a Somatom Sensation 16 MDCT scanner (Siemens, Forchheim, Germany) (n = 15). The examinations on the 4-detector MDCT were performed with 120 kV, 240 mAs, a table speed of 7.5 mm per rotation, high-quality mode, a pitch of 3:1, and the scans were reconstructed at a 3-mm interval. The examinations at the 16-detector MDCT were performed with 16 × 0.75 mm collimation and 0.5s rotation speed, 120 kV, 140 effective mAs, 12 mm/rotation table-feed, and the images were reconstructed with a 3 mm increment and 3 mm thickness. All the scans were acquired in the expiratory breathhold during IV injection of 120-150 mL of iopamidol (Iopamiro; Bracco, Milano, Italy) at a concentration of 370 mg I/mL or iohexol 60% (Omnipaque 300; Nycomed Amersham, Oslo, Norway) at a concentration of 300 mg I/mL at a rate of 3-4 mL/sec with using a power injector (EnVision CT; Medrad, Pittsburgh, PA). A 2 mL/kg dose of the contrast media was administered to patients with the body weight of 60-75 kg. The total dose was fixed at 150 mL for the patients with the body weight more than 75 kg and at 120 mL for the patients with the body weight less than 60 kg. The scan delay of the first AP scan was determined by the bolus tracking software, and it was generally 33-40 sec after the beginning of the injection, according to the time to the aortic enhancement of the 100 HU. The second PP scan was obtained at the time delay of 20 sec after the first acquisition on the 4-detector CT, and 24 seconds on the 16-detector CT. The upper abdomen was scanned in the AP scan and the whole abdomen with the pelvic cavity was imaged in the PP scan with a craniocaudal orientation.
Two abdominal radiologists with four and six years of experience, respectively, in abdominal imaging independently and retrospectively reviewed the PP images (the PP only set), at first at random, without knowing of the final clinical diagnosis. Next, they reviewed the AP images combined with the PP images (the AP plus PP set). To minimize the recall bias, the two interpretation sessions were separated by at least two weeks. The technical parameters used and the patient identifiers were concealed from the reviewers at the time of the interpretation. They were allowed to choose the window width and the window level for each image as they found appropriate. The reviewers assigned each image the confidence rating by using a 5-grade scale (1 = definitely no; 2 = probably no; 3 = possibly no; 4 = probably yes; 5 = definitely yes) for the following items; a) the presence of CT signs of PID on the PP image; b) the probability of FHCS as the diagnosis based on the degree of the increased perihepatic enhancement on each image set. The positive result was defined as grade 4 and 5 and the negative result was graded as 1, 2 or 3. The inclusion criteria of the imaging findings for the diagnosis of PID in this study were as follows: inflammatory engorgement of the cervix, ovaries or fallopian tubes, endometrial thickening, pyosalpinx, enhanced thickening of the fallopian tubes filled with complex fluid and debris, loculated pelvic fluid or ascites, and tubo-ovarian and pelvic abscesses (16).
The final clinical diagnosis provided confirmation of the individual diagnosis in this study. The image interpretations of the different sets of the images were compared with the final clinical diagnosis to estimate the diagnostic accuracy. To compare the individual diagnostic accuracy of FHCS of both the different sets of the images the receiver operating characteristic (ROC) analysis was used (17). The McNemar test (a value of less than 0.05 was considered statistically significant) was used to compare the sensitivity for the diagnosis of FHCS for each reader on the both sets of images. To assess the degree of the interobserver agreement of each item, the weighted kappa (wk) of each item was calculated using the data obtained by the two independent radiologists (18). In this study, the weighted kappa was classified as poor (0.21-0.40), moderate (0.41-0.60) and substantial agreement (0.61-0.80) (18).
For the both readers, the accuracy (Az value) of the diagnosis of FHCS was higher with the AP plus the PP image set (Az, 0.905; 95% CI, 0.808-1.000 for reader 1; Az, 0.942;95% CI, 0.882-1.000 for reader 2) as compared with using only the PP image set (Az, 0.806; 95% CI, 0.683-0.930 for reader 1; Az, 0.706; 95% CI, 0.562-0.849 for reader 2) (Fig. 1). The difference in the diagnostic accuracy between the AP plus PP image set and using only the PP image set was statistically significant for reader 2 (p = 0.0003), and it nearly approached statistical significance for reader 1 (p = 0.0516). The overall false positive and (false) negative results, specificities, PPVs and NPVs on the both image sets for each reader are presented in Table 1. When an overall grade 3 for the visual grade was used as the cut-off value for the diagnosis of FHCS, the sensitivities were significantly higher (p = 0.000 for the both readers; McNemar test) with the AP plus the PP image set (88% for the both readers) than with using only the PP image set (28% for reader 1 and 4% for reader 2) (Fig. 2).
The increased perihepatic enhancement was perceived by the both readers on both image sets in one patient (Fig. 3). One false positive diagnosis was made by the both readers on the AP plus PP image set. This patient presented with diffuse abdominal pain and the patient showed the increased perihepatic enhancement on the AP image with pelvic fat infiltration (Fig. 4). The patient was clinically diagnosed with mild PID because a mild fluid collection was found on the pelvic ultrasonography performed by the gynecologist with the absence of other clinical or laboratory findings indicative of FHCS. Three more false positive results were made by reader 2 on the interpretation of the AP plus PP image set. Clinically, these patients were diagnosed with PID, but they did not complain of RUQ pain, and no other evidence of FHCS was found. Three false negative diagnoses were made by both readers on the interpretation of the AP plus PP image set. Two patients showed diffuse heterogenous enhancement of the entire liver (Fig. 5), which was considered to be intrahepatic pathology rather than perihepatic enhancement caused by FHCS. However, those patients were clinically diagnosed as FHCS and they were given antibiotic treatment. The abnormal liver enhancement disappeared on the follow up CT two weeks later in one patient, and the other patient was not taken for the follow up CT. In another patient, both readers did not consider that increased perihepatic enhancement was present. The patient had a history of RUQ pain one month prior to the CT examination and had undergone antibiotic treatment.
The interobserver agreement for the probability of FHCS as a diagnosis based on the degree of the increased perihepatic enhancement was substantial on the AP plus PP image set (wk = 0.719), but it was moderate on only the PP image set (wk = 0.413). The interobserver agreement for the presence of PID was moderate (wk = 0.53) on only the PP image set (Table 2).
The "violin string-like adhesions" on laparoscopic examination is considered a finding characteristic of FHCS, especially in the chronic phase of the disease (19, 20). However, the "violin string-like adhesions" may be also seen by laparoscopy in familial Mediterranean fever and diaphragmatic endometriosis (21, 22). Sonography may demonstrate the "violin string" (8), but widening of the right anterior renal space and loculation of fluid in the hepatorenal space are the usual findings (5-8, 23). CT may also be helpful to demonstrate the "violin-string appearance" (24). Hepatic capsular enhancement due to an increased blood flow or inflammation at the hepatic capsule was recently reported as a characteristic feature of the acute phase of FHCS on contrast enhanced CT scans for the diagnosis of FHCS (10, 11). The characteristic laparoscopic findings of the acute phase is moist inflammation with injection of the vessels and exudate formations on the anterior surface of the liver and the peritoneal surface of the abdominal wall (19), and this may contribute to the increased perihepatic enhancement during the acute phase (11). The proposed mechanisms for the pathway of the disease spread from the pelvic inflammation to the subphrenic or perihepatic region are as follows: 1) transperitoneal ascending spread of the inflammation from the pelvis along the bilateral paracolic gutters, according to the ascitic fluid flow, especially on the right side; 2) hematogenous spread; 3) translymphatic spread; and 4) an exaggerated immune response (1, 19, 25, 26). The results of our study show that inclusion of the AP scan dramatically improves the diagnostic accuracy in FHCS patients by increasing the depiction of the increased perihepatic enhancement.
Considering the marked increase of the false negative results by the both readers on only the PP image set, as compared with the AP plus PP set, we do believe that the estimation of the increased perihepatic enhancement on the portal image is appropriate. In this study, there were 22 true positive results on the AP plus PP image set by the both readers, and the mean period between symptom onset and the CT scan in the group of FHCS patients was 2±0.72 days. This may imply that the increased perihepatic enhancement could be a sign of the acute phase inflammation. There was one patient in whom both the readers distinctly noted the increased perihepatic enhancement on the PP image as well as on the AP image; the interval between the onset of the symptom and the time of the CT examination in this patient was 10 days. In this case, the relatively long duration of the inflammation might have contributed to the visualization of the increased perihepatic enhancement on both the AP and PP images, but this may also be associated with relatively poor enhancement of the liver on the PP images because of the delayed circulation in this patient. One false positive result was made by both readers on the AP plus PP image set in this study. The patient presented with diffuse abdominal pain, normal laboratory findings and minimal fluid collection in the pelvic cavity on gynecological US; the final clinical diagnosis was mild PID. However, we could not completely exclude the possibility of FHCS that was not confirmed by the appropriate laboratory findings.
Increased perihepatic enhancement has also been noted in other conditions such as perihepatitis associated with systemic lupus erythematous (SLE) (27), liver abscess, cholangitis, peritoneal carcinomatosis, tuberculous or other peritonitis, acute cholecystitis, superior vena cava obstruction, congenital hepatic fibrosis or vascular variations such as capsular veins and aberrant veins (28-34). However, other image findings are noted in most of those conditions, and these additional findings can be helpful for making a correct diagnosis. Therefore, increased perihepatic enhancement in the absence of other liver or peritoneal diseases can be a relevant finding for the radiological diagnosis of FHCS in female patients, especially when it is associated with clinical or imaging findings of PID and RUQ pain.
Atypical imaging features of FHCS, such as a large loculated perihepatic fluid collection or a transient hepatic attenuation difference and gallbladder wall thickening have been reported (12, 23). Mild gallbladder wall thickening was also noted in two patients in our study. In two patients in our study, heterogeneously increased enhancement was not confined to the perihepatic area, but it extended to the entire liver. Both the readers considered these findins to be associated with ntrahepatic pathology rather than with FHCS. However, these cases presented no evidence of the primary liver disease and sustained typical clinical manifestations of FHCS, and the symptoms improved after the antibiotic treatment and the cases were finally diagnosed as FHCS with bilateral tubo-ovarian abscess or PID. Our results suggest that the diffuse hepatic enhancement may occur in cases of FHCS as well as increased perihepatic enhancement. In one false negative case, the both readers failed to observe the increased perihepatic enhancement. This patient had a history of admission to another hospital for identical manifestations one month earlier. At the time of admission in our hospital, her symptoms and signs were compatible with FHCS with PID. In this case, recurring or chronic manifestations of FHCS might have contributed to the false negative result.
Neisseria gonorrhoeae and Chlamydia trachomatis have been reported as common causative organisms of FHCS, however; chlamydia infection was the main pathogenic agent in the vast majority of cases (4, 35, 36) in our studies. FHCS associated with other pathogens such as Mycobacterium tuberculosis has also been reported (37), and gonorrheal infection may also cause FHCS in male patients (25). In the present study, 20 of 25 patients were positive for chlamydial infection, 2 were negative and 3 were not tested. They were negative for Neisseria gonorrhoeae, but there were many Gram-positive cocci in the cervical culture in the two patients who were negative for the chlamydial infection, and they had typical RUQ pain and the symptoms or signs of salpingitis on the gynecological examination.
Our study has certain drawbacks. First, none of the patients of FHCS underwent laparoscopical examination. Currently, the diagnosis of FHCS is usually made based on clinical and laboratory findings; thus, the identification of appropriate findings on CT will be helpful for the correct diagnosis and management. Second, to avoid the bias for the evaluation of the perihepatic enhancement, we excluded patients that presented with CT findings of other diseases such as acute cholecystitis, appendicitis and the obvious findings of acute pyelonephritis. Patients who presented with ambiguous clinical and imaging findings were included in the control group, and the causes of their abdominal pain were not identified in many of them. Therefore, we can not exclude the possibility that undiagnosed patients with FHCS may have been included in the control group. However, no patients in the control group developed any signs of FHCS or other complications. Third, almost all the patients had the acute phase of FHCS, so this study does not reflect the chronic phase.
In conclusion, our study has demonstrated that by using a biphasic CT examination, including an AP scan obtained at the optimum temporal window, the depiction of the increased perihepatic enhancement can be significantly improved. Using this technique, the sensitivity and the accuracy of diagnosing FHCS can be markedly increased during the evaluation of patients with acute RUQ pain or if there is the suspicion of FHCS.
Figures and Tables
Fig. 1
Receiver operating characteristic curves for reader 1 (A) and reader 2 (B) for the diagnosis of Fitz-Hugh-Curtis syndrome. The accuracy of the arterial phase plus portal venous phase set showed high statistical significance compared with using only the portal venous phase set for reader 2 (p = 0.0003) (B), and it nearly approached statistical significance for reader 1 (p = 0.0516) (A). The overall accuracy was superior for the arterial phase plus portal venous phase image set in respect to using only the portal venous phase set.

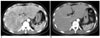
Fig. 2
Axial contrast-enhanced CT scan in a 24-year-old woman with right upper quadrant pain and fever, which is a true positive example of Fitz-Hugh-Curtis syndrome.
A. The arterial phase scan reveals conspicuous homogenously increased perihepatic enhancement on the right lobe of the liver.
B. Portal venous phase scan reveals inconspicuous enhancement.
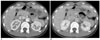
Fig. 3
Axial contrast-enhanced CT scan in a 40-year-old woman with right upper quadrant pain and fever, which is a true positive example of Fitz-Hugh-Curtis syndrome.
A. Arterial phase scan reveals conspicuous increased perihepatic enhancement on the right lobe of the liver.
B. Portal venous phase scan reveals conspicuous identical enhancement.
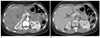
Fig. 4
Axial contrast-enhanced CT scan in a 25-year-old woman with mild pelvic inflammatory disease as the final diagnosis, which is a false positive example of Fitz-Hugh-Curtis syndrome.
A. Arterial phase scan reveals the increased perihepatic enhancement at the anterior portion of the right lobe of the liver.
B. Perihepatic enhancement cannot be seen on the portal venous phase scan.
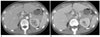
Fig. 5
Axial contrast-enhanced CT scan in a 49-year-old woman with right upper quadrant pain and fever, which is a false negative example of Fitz-Hugh-Curtis syndrome.
A. Arterial phase scan reveals a diffuse heterogenous perihepatic enhancement on the entire liver.
B. Follow up arterial phase scan 2 weeks later reveals no enhancement.
References
1. Peter NG, Clark LR, Jaeger JR. Fitz-Hugh-Curtis syndrome: a diagnosis to consider in women with right upper quadrant pain. Cleve Clin J Med. 2004. 71:233–239.
2. Counselman FL. An unusual presentation of Fitz-Hugh-Curtis syndrome. J Emerg Med. 1994. 12:167–170.
3. Paavonen J, Saikku P, von Knorring J, Aho K, Wang SP. Association of infection with Chlamydia trachomatis with Fitz-Hugh-Curtis syndrome. J Infect Dis. 1981. 144:176.
4. Litt IF, Cohen MI. Perihepatitis associated with salpingitis in adolescents. JAMA. 1978. 240:1253–1254.
5. Dinerman LM, Elfenbein DS, Cumming WA. Clinical Fitz-Hugh-Curtis syndrome in an adolescent. Ultrasonographic findings. Clin Pediatr (Phila). 1990. 29:532–535.
6. Banerjee B, Rennison A, Boyes BE. Sonographic features in a case of Fitz-Hugh-Curtis syndrome masquerading as malignancy. Br J Radiol. 1992. 65:342–344.
7. Schoenfeld A, Fisch B, Cohen M, Vardy M, Ovadia J. Ultrasound findings in perihepatitis associated with pelvic inflammatory disease. J Clin Ultrasound. 1992. 20:339–342.
8. van Dongen PW. Diagnosis of Fitz-Hugh-Curtis syndrome by ultrasound. Eur J Obstet Gynecol Reprod Biol. 1993. 50:159–162.
9. Piscaglia F, Vidili G, Ugolini G, Ramini R, Montroni I, De Iaco P, et al. Fitz-Hugh-Curtis-syndrome mimicking acute cholecystitis: value of new ultrasound findings in the differential diagnosis. Ultraschall Med. 2005. 26:227–230.
10. Nishie A, Yoshimitsu K, Irie H, Yoshitake T, Aibe H, Tajima T, et al. Fitz-Hugh-Curtis syndrome. Radiologic manifestation. J Comput Assist Tomogr. 2003. 27:786–791.
11. Tsubuku M, Hayashi S, Terahara A, Furukawa T, Ohmura G. Fitz-Hugh-Curtis syndrome: linear contrast enhancement of the surface of the liver on CT. J Comput Assist Tomogr. 2002. 26:456–458.
12. Pickhardt PJ, Fleishman MJ, Fisher AJ. Fitz-Hugh-Curtis syndrome: multidetector CT findings of transient hepatic attenuation difference and gallbladder wall thickening. AJR Am J Roentgenol. 2003. 180:1605–1606.
13. Mesurolle B, Mignon F, Gagnon JH. Fitz-Hugh-Curtis syndrome caused by Chlamydia trachomatis: atypical CT findings. AJR Am J Roentgenol. 2004. 182:822–824. author reply 824.
14. Chevalier N, De Tayrac R, Dagher I, Mockly JF, Franco D, Fernandez H. [Peri-hepatitis abscess secondary to pelvic peritonitis]. J Gynecol Obstet Biol Reprod (Paris). 2002. 31:681–683.
15. Marbet UA, Stalder GA, Vogtlin J, Loosli J, Frei A, Althaus B, et al. Diffuse peritonitis and chronic ascites due to infection with Chlamydia trachomatis in patients without liver disease: new presentation of the Fitz-Hugh-Curtis syndrome. Br Med J (Clin Res Ed). 1986. 293:5–6.
16. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002. 22:1327–1334.
17. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983. 148:839–843.
18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.
19. Lopez-Zeno JA, Keith LG, Berger GS. The Fitz-Hugh-Curtis syndrome revisited. Changing perspectives after half a century. J Reprod Med. 1985. 30:567–582.
20. Watanabe M, Tanaka S, Ono M, Hamamoto S, Niigaki M, Uchida Y, et al. Laparoscopic observations of hepatic capsular abnormalities: non-postoperative adhesions and hepatic capsular thickening. Gastrointest Endosc. 1999. 50:664–666.
21. Romero-Gomez M, Corpas R, Sanchez-Munoz D, Grande L, Caballero V. Familial Mediterranean fever mimicking Fitz-Hugh-Curtis syndrome. Am J Gastroenterol. 2003. 98:701.
22. Takeuchi H, Kitade M, Sakurai A, Kikuchi I, Kumakiri J, Kinoshita K. Fitz-Hugh and Curtis syndrome-like diaphragmatic endometriosis. Fertil Steril. 2005. 83:1039–1040.
23. Romo LV, Clarke PD. Fitz-Hugh-Curtis Syndrome: pelvic inflammatory disease with an unusual CT presentation. J Comput Assist Tomogr. 1992. 16:832–833.
24. Haight JB, Ockner SA. Chlamydia trachomatis perihepatitis with ascites. Am J Gastroenterol. 1988. 83:323–325.
25. Fung GL, Silpa M. Fitz-Hugh and Curtis syndrome in a man. JAMA. 1981. 245:128.
26. Kimball MW, Knee S. Gonococcal perihepatitis in a male. The Fitz-Hugh-Curtis syndrome. N Engl J Med. 1970. 282:1082–1084.
27. Schoenwaelder M, Stuckey SL. Perihepatitis associated with systemic lupus erythematosus: computed tomography findings. Australas Radiol. 2005. 49:179–181.
28. Chen WP, Chen JH, Hwang JI, Tsai JW, Chen JS, Hung SW, et al. Spectrum of transient hepatic attenuation differences in biphasic helical CT. AJR Am J Roentgenol. 1999. 172:419–424.
29. Yamashita K, Jin MJ, Hirose Y, Morikawa M, Sumioka H, Itoh K, et al. CT finding of transient focal increased attenuation of the liver adjacent to the gallbladder in acute cholecystitis. AJR Am J Roentgenol. 1995. 164:343–346.
30. Itai Y, Murata S, Kurosaki Y. Straight border sign of the liver: spectrum of CT appearances and causes. Radiographics. 1995. 15:1089–1102.
31. Rodriguez E, Pombo F. Peritoneal tuberculosis versus peritoneal carcinomatosis: distinction based on CT findings. J Comput Assist Tomogr. 1996. 20:269–272.
32. Quiroga S, Sebastia C, Pallisa E, Castella E, Perez-Lafuente M, Alvarez-Castells A. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics. 2001. 21:65–81. questionnaire 288-294.
33. Yilmaz T, Sever A, Gur S, Killi RM, Elmas N. CT findings of abdominal tuberculosis in 12 patients. Comput Med Imaging Graph. 2002. 26:321–325.
34. Zeitoun D, Brancatelli G, Colombat M, Federle MP, Valla D, Wu T, et al. Congenital hepatic fibrosis: CT findings in 18 adults. Radiology. 2004. 231:109–116.
35. Muller-Schoop JW, Wang SP, Munzinger J, Schlapfer HU, Knoblauch M, Tammann RW. Chlamydia trachomatis as possible cause of peritonitis and perihepatitis in young women. Br Med J. 1978. 1:1022–1024.
36. Katzman DK, Friedman IM, McDonald CA, Litt IF. Chlamydia trachomatis Fitz-Hugh-Curtis syndrome without salpingitis in female adolescents. Am J Dis Child. 1988. 142:996–998.
37. Sharma JB, Malhotra M, Arora R. Fitz-Hugh-Curtis syndrome as a result of genital tuberculosis: a report of three cases. Acta Obstet Gynecol Scand. 2003. 82:295–297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download