This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
We wanted to investigate the ability of breast MR imaging to identify the primary malignancy in patients with axillary lymph node metastases and initially negative mammography and sonography, and we correlated those results with the conventional imaging.
Materials and Methods
From September 2001 to April 2006, 12 patients with axillary lymph node metastases and initially negative mammography and sonography underwent breast MR imaging to identify occult breast carcinoma. We analyzed the findings of the MR imaging, the MR-correlated mammography and the second-look sonography. We followed up both the MR-positive and MR-negative patients.
Results
MR imaging detected occult breast carcinoma in 10 of 12 (83%) patients. Two MR-negative patients were free of carcinoma in the ipsilateral breast during their follow-up period (39 and 44 months, respectively). In nine out of 10 patients, the MR-correlated mammography and second-look sonography localized lesions that were not detected on the initial exam. All the non-MR-correlated sonographic abnormalities were benign.
Conclusion
Breast MR imaging can identify otherwise occult breast cancer in patients with metastatic axillary lymph nodes. Localization of the lesions through MR-correlated mammography and second-look sonography is practically feasible in most cases.
Keywords: Axilla, Breast, MR, Breast neoplasms, Lymph nodes
The manifestation of an isolated metastatic axillary lymph node without any clinical and radiologic evidence of primary breast cancer is uncommon, but this disease most commonly originates from breast cancer in women (
1). For these cases, the traditional therapy has been upper outer quadrantectomy or blind mastectomy with axillary lymph node dissection, with or without radiation (
2). However, 30-40% of the upper outer quadrantectomy or blind mastectomy specimens show no histologic evidence of carcinoma, and the survival rate of patients who undergo blind mastectomy with axillary dissection is similar to those who undergo axillary dissection only (
3,
4). Breast MRI techniques have improved significantly in recent years, and MRI with dynamic enhancement has been established as an important imaging modality for evaluating breast cancer with the highest sensitivity (
5). Therefore, the goal of this study was to investigate the ability of breast MR imaging to identify the primary malignant focus in patients with metastatic axillary lymph nodes and initially negative mammography and sonography, and to correlate the results with the conventional imaging.
MATERIALS AND METHODS
Patients
From September 2001 to April 2006, 26 patients presenting with metastatic axillary lymph nodes and without any clinical evidence of breast cancer visited our hospital. In 11 cases, primary breast cancers were detected in the ipsilateral breasts by mammography (n = 3) or US (n = 7) or both (n = 1). Two cases had a history of contralateral breast cancer and they had received modified radial mastectomy and chemotherapy three and four years ago, respectively. In one case, the diagnosis of the core needle biopsy of the axillary lymph node was metastatic poorly differentiated carcinoma or medullary carcinoma, but the final diagnosis after axillary lymph node dissection was anaplastic large cell lymphoma. The remaining 12 patients, who showed no evidence of primary breast cancer upon clinical examination or on the initial mammography and sonography, underwent breast MR imaging to evaluate the presence of occult breast carcinoma; it was these 12 patients who made up the study population. All 12 patients had malignant axillary lymph nodes that were pathologically shown to be metastatic adenocarcinomas by ultrasound (US)-guided core needle biopsy (n = 11) and excision biopsy (n = 1). All these patients were female and they ranged in age from 42 to 78 years (mean age: 55 years).
All the available images of the patients from the referring hospital, including 10 mammograms, nine US and one PET, were negative. Ten patients had both negative mammograms and US; two patients had negative mammograms and US with probable benign nodules.
Breast MR Imaging
All the patients were imaged in the prone position using a dedicated surface breast coil. Breast MR imaging was performed on a 1.5-T system (Signa CV/I; General Electric Medical System, Milwaukee, WI) for eight cases and on a 3.0-T system (Interna; Philips Medical Systems, Best, the Netherlands) for three cases, and on both systems for one case. MR imaging consisted of a fat-suppressed, sagittal, 3-dimensional, gradient echo sequence and the dynamic enhanced images. Imaging on the 1.5-T scanner covered a single breast with a minimum repetition time and echo time (17.3/1.3 ms), a 60° flip angle, a 24-cm field of view, 1-mm to 2-mm sections with no gap, a 256×192 matrix, one excitation and a scan time of 3-4 minutes. Imaging on the 3.0-T scanner covered both breasts with a minimum repetition time and echo time (8.7/4.3 ms for the axial dynamic images; 16/4.1 ms for the sagittal dynamic images), a 20° flip angle, a 27-cm field of view, 1.5-mm sections with no gap, a 512×512 matrix and a scan time of 2-3 minutes.
For the dynamic contrast enhancement, a 0.1 mmol/kg bolus of gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ) was injected; this was followed by a 10-mL saline flush. Two and three sequential postcontrast images were obtained with no delay on the 1.5-T and 3.0-T scanners, respectively, and these began immediately after the saline flush at the same slice position and location. After examination, two subtraction images were made automatically on a pixel-by-pixel basis: the un-enhanced images were subtracted from the early postcontrast images (standard subtraction), and the last postcontrast images were subtracted from the early postcontrast images (reverse subtraction). The reformatted images with a maximum intensity projection were then created from the standard and reverse subtraction images.
Interpretation of the Breast MR Images
The MR images were interpreted prospectively on soft copy with using PACS (picture archiving and communication system, General Electric Medical System) that allowed manual window settings and optimization of the parameters. A radiologist, who was aware of the patient's clinical history and who had seen all available imaging information, including the mammograms and sonograms, interpreted all the images. The breast lesions were described using the Breast Imaging Reporting and Data System (BI-RADS) MR lexicon (
6).
A lesion was considered suspicious if it was visualized as (a) a mass with irregular shape, a mass with irregular or spiculated margin, or a mass with rim enhancement, (b) non-mass-like enhancement that showed a clumped linear-ductal enhancement, clumped segmental enhancement and regional enhancement with ill-defined or irregular borders or with architectural distortion, or (c) a mass or non-mass-like enhancement with early washout.
Management of Lesions after MRI
The previous mammography and US images were retrospectively reviewed along with any information gleaned from the MR images; any positive findings, including benign or probable benign lesions in the areas corresponding to suspicious enhancements on the MRI, were confirmed by US-guided or mammography-guided biopsy. If retrospective review of the previous mammography or US images was not available or if this was negative, then MR-correlated mammography or a second-look US examination that specifically targeted the area of suspicious MR enhancement was performed. The benign results from the US-guided or mammography-guided biopsy in the area of suspicious MR enhancement were followed by localization with excision biopsy.
Since MR-guided localization and biopsy were not available at our institution, those lesions with suspicious enhancement on MRI, but with no abnormality on mammography or the US images, underwent mastectomy.
RESULTS
Suspicious lesions were detected on breast MRI in 10 (83%) of the 12 patients. The pathology of all the lesions was proven to be primary breast carcinoma.
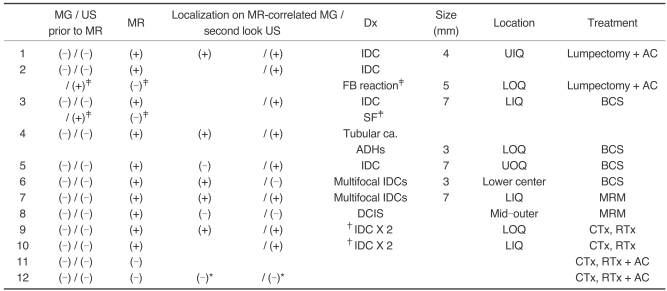
A complete summary of the 12 patients presenting with axillary lymph node metastases is given in
Table 1. All the patients had mammography and US examinations conducted before undergoing breast MRI. All 12 patients were transferred from other hospitals with negative mammography and US images or they were transferred without these images. Seven of them again received mammography and US examinations at our hospital, and the other five of them were diagnosed by the images that were obtained from other hospitals.
Before MR imaging, 10 out of the 12 patients had no abnormal findings based on the conventional images, while two patients had category 3 nodules observed on US. These two category 3 nodules did not correlate with the MR findings and they were diagnosed as a foreign body reaction and stromal fibroses by US-guided core needle biopsy.
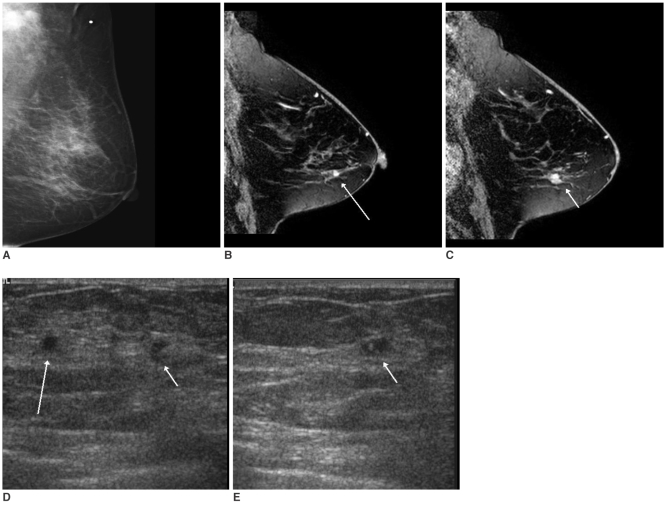
MRI of the breast showed suspicious lesions in 10 patients, and these lesions had been negative on the prior conventional images. MR-guided second-look US examinations were performed in each of those 10 patients, as well as performing mammography in four patients. Subsequent localization of the lesions detected on MRI was possible on mammography (n = 1), US (n = 4) or on both examinations (n = 4) in nine out of 10 patients. In three patients, the lesions localized on MR-guided second-look examinations were category 4 there were two cases of suspicious nodules less than 1 cm in size on US (
Fig. 1) and one case of faint pleomorphic calcifications with a segmental distribution on the mammogram (
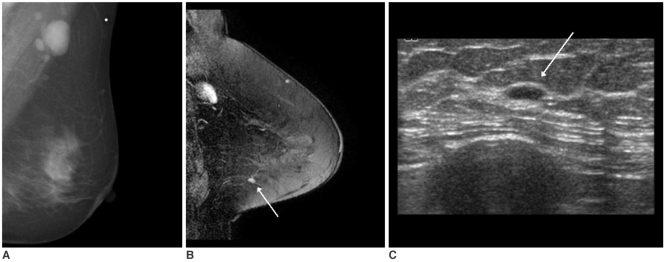
Fig. 2). Category 3 lesions were found in five patients with 3-mm to 8-mm sized benign-looking nodules being seen on US (
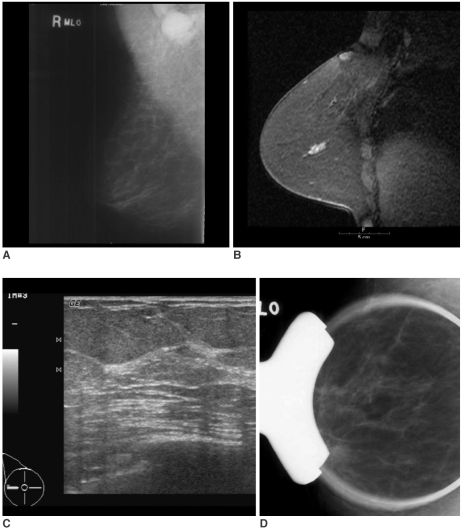
Fig. 3). A category 2 lesion was found in one patient who had a few benign cysts (
Fig. 4). All the lesions localized on the MR-correlated mammography or the MR-guided second-look US images were found to be malignant after US-guided core needle biopsy (n = 5), US-guided fine needle aspiration (n = 1), surgical excision after US-guided localization (n = 2) or after mammography-guided localization (n = 1). The MR-guided second-look examination failed to localize the lesion in one patient who had a 1.8-cm linear non-mass-like enhancement with focal wash out on MRI. This lesion was found to be ductal carcinoma in situ (DCIS) after performing modified radical mastectomy (
Fig. 5).
After histologic confirmation of the primary breast malignancies for 10 MR-detected lesions, two patients underwent lumpectomy following the US-guided localization, four patients were treated with breast conserving surgery (BCS) after US or mammography-guided localization, and one patient underwent modified radical mastectomy (MRM) for multifocal lesions. As noted above, the patient who had a suspicious lesion observed on MRI that was not otherwise localized, this patient received MRM as well. Two patients were treated with chemotherapy and radiation therapy without surgical intervention due to their multifocal breast cancers with distant metastases.
Of the 10 cases that underwent surgical treatment or biopsy, five cases were invasive ductal carcinomas, one was tubular carcinoma and one was ductal carcinoma in situ. Four of the patients with invasive ductal carcinoma had multiple lesions, and the patient with tubular carcinoma had multiple areas of atypical ductal hyperplasia around the carcinoma. The mean size of the surgically removed invasive carcinomas was 4.8 mm (range: 3-7 mm).
In two patients with negative findings on both MRI and the conventional images, there was still no evidence of the primary malignancy in the ipsilateral breast on the conventional images or on the follow-up MRI during the follow-up periods of 39 and 44 months, respectively.
DISCUSSION
With its recent technical advances, contrast-enhanced MRI plays an important role in identifying primary breast cancer, for defining the extent of tumor and for helping decide the therapeutic plans (
1). While mammography is the primary imaging modality used to evaluate breast lesions, the false negative rate for mammography has been reported to be about 30% (
7,
8). The sensitivity of mammography is especially limited when the lesion is within dense breast parenchyma, and it is often as low as 45% (
9). Among mammography, US and MRI, MRI is the most sensitive method for evaluating breast cancer and it is well recognized that MRI can detect otherwise occult breast cancer (
5,
9,
10).
A few previous reports that detailed the MR images of patients with axillary lymph node metastases, and the patients had prior negative clinical and mammography findings, demonstrated 36%, 75% and 86% rates of detecting cancer by breast MRI, respectively (
1,
11,
12). In another previous study on patients with axillary lymph node metastasis and negative clinical, mammographic and US findings, the investigators showed a lesion detection rate of 64% with breast MRI, and 67% of the detected lesions were found to be primary breast cancer (
13). The reported mean size of the lesions visible by breast MRI alone was 14.6 mm (
14), while the mean lesion size detected by MRI in our study was 4.8 mm.
The results of mammography and US depend on the quality of the mammogram and the persons who interpret the mammogram and perform the US examination. In our study, when the quality of the initial mammogram from other hospital was not satisfactory, we then performed mammography of the ipsilateral breast again for the same patient. Two radiologists interpreted all the mammograms and one of them was a board-certified experienced radiologist who had worked in the field of breast imaging for more than 10 years. More than six persons performed this study's US examinations, from a resident with two years experience in US examination to a board certified experienced radiologist with more than 10 years experience in breast US. This can be the limitation of our study, but the lesions detected by MRI alone were smaller than those detected by MRI in the reported cases (
14).
In our study, breast MRI showed suspicious lesions in 83% (10/12) of the patients who had prior negative clinical, mammography and US findings. Instead of performing the traditional blind mastectomy or upper outer quadrantectomy, eight patients received surgery: two lumpectomies, four BCSs and two MRMs. In addition, two patients were treated with chemotherapy and radiation therapy based on the information regarding the site and extent of their primary malignancy. As a result of the confirmed information about the lesions, all the patients were treated appropriately and conservatively, with the exception of one patient who received MRM for a suspicious lesion on MRI that was not otherwise localized. It is worthwhile to note that six patients were able to avoid unnecessary mastectomy or upper outer quadrantectomy, as the primary breast carcinoma was located in the upper outer quadrant in only one patient.
The one case that was localized only on MRI and the patient underwent MRM was pure DCIS. Historically, the reported incidence of axillary metastasis in patients with DCIS is 1-2%, but the prevalence of positive lymph nodes in patients with pure DCIS is approximately 2-13% with performing immunohistochemical staining (
15,
16). Node metastasis is usually observed in the sentinel lymph node only in patients with DCIS (
17), but in our case, four lymph nodes were revealed to have malignant cells after MRM.
Since MRI has a very low false-negative rate (
9,
15), we did not perform blind mastectomy or quadrantectomy in the two patients who had negative findings on MRI. They have shown no evidence of malignancy in their breasts during the follow-up period (39 and 44 months, respectively) to date.
Therefore, we suggest that before developing a therapeutic plan, breast MRI must be performed for those patients with axillary lymph node metastasis and who are without evidence of primary breast cancer.
Although contrast-enhanced MRI has the highest sensitivity among the many imaging modalities for the breast, the specificity of breast MRI is low (
9,
10,
16). Therefore, preoperative biopsy or localization of the MR-detected lesion is necessary (
17). Yet MR imaging-guided biopsy or localization requires commercially available MRI guiding equipment. Also, the cost-effectiveness of MRI-guided biopsy or localization has not yet been fully assessed (
18). Even when MRI guiding equipment is available, lesions in the medial breast are difficult to access and small enhancing lesions (around 5 mm) may be difficult to localize due to the transient nature of contrast enhancement and the obscuring that occurs when placing the biopsy needle. MR imaging-guided biopsy or localization is also a time-consuming procedure that lasts as long as 45 to 60 minutes (
14,
17,
18).
A few studies have reported excellent performance of MR-guided second-look US localization (
11,
19). In our study, MR-guided second-look examinations localized all the lesions that were detected on breast MRI alone, with the exception of one case of DCIS. Therefore, localization with using MR-correlated mammography or MR-guided second-look US can be a cheaper, practical imaging alternative.
In our experience, the lesions that were negative on prior US or mammography, but that were localized on MR-correlated mammography or second-look sonography, included not only suspicious malignant lesions, but also benign-looking lesions (five category 3 lesions and one category 2 lesion) that were small in size; all of these were found to be malignant. However, two category 3 nodules that were detected on prior US, but that did not correlate with the MR findings, were revealed as benign lesions after biopsy. When BI-RADS category 2 or 3 lesions are detected on conventional images, but they do not correlate with the MR findings of the patients with suspicious occult breast cancer, then we should not neglect these lesions. However, biopsy for all benign-looking lesions is not desirable or even necessarily meaningful (
6). Based on our experience, we suggest that when there is a benign or benign-looking lesion on the conventional images of the patients with metastatic axillary lymph node(s) and an unknown primary malignancy, then the conventional images should first be correlated with MR findings before performing a biopsy of the lesions.
In conclusion, the high sensitivity of contrast-enhanced breast MRI played an essential role in evaluating occult breast cancer in patients who presented with a metastatic axillary lymph node with an unknown primary malignancy, and contrast-enhanced breast MRI also played an essential role for determining the therapeutic plan. In 90% of our cases, histologic work-up for the lesions detected on the breast MRI was practically feasible with successful localization being achieved under MR-guided second-look US or MR-correlated mammography.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download