Abstract
Objective
To determine the optimal threshold for the attenuation values in unenhanced computed tomography (CT) and assess the value of the size criteria for differentiating between an adrenal adenoma and a nonadenoma.
Materials and Methods
The unenhanced CT images of 45 patients at our institution, who underwent a surgical resection of an adrenal masses between January 2001 and July 2005, were retrospectively reviewed. Forty-five adrenal masses included 25 cortical adenomas, 12 pheochromocytomas, three lymphomas, and five metastases confirmed by pathology were examined. The CT images were obtained at a slice thickness of 2 mm to 3 mm. The mAs were varied from 100 to 160 and 200 to 280, while the 120 KVp was maintained in all cases. The mean attenuation values of an adrenal adenoma and nonadenoma were compared using an unpaired t test. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy at thresholds of 10 HU, 20 HU, and 25 HU were compared. The diagnostic accuracy according to the size criteria from 2 cm to 6 cm was also compared.
Results
The twenty-five adenomas showed significantly lower (p < 0.05) attenuation values(mean ± SD; 16.3 ± 14.9) than the nonadenomas (38.1 ± 6.8). Nineteen (90%) of the 20 nonadenomas had attenuation values ranging from 30 to 50 HU. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for diagnosing adenomas were 36%, 100%, 100%, 56%, and 64%, respectively, at a threshold of 10 HU; 60%, 100%, 100%, 67%, and 78%, respectively, at a threshold of 20 HU; and 72%, 95%, 95%, 73%, and 82%, respectively, at a threshold of 25 HU. The adenomas had a significantly (p < 0.05) smaller diameter (2.44 ± 1.24 cm) than the nonadenomas (5.09 ± 2.37 cm). The size criteria using a diameter of 4-6 cm showed a sensitivity > 90% but a specificity < 70%. Size criteria of 2 or 3 cm had a high specificity of 100% and 80% but a low sensitivity of 20% and 60%.
Adrenal masses are found in approximately 5-10% of patients in imaging procedures performed for reasons unrelated to the adrenal glands (1-3). The reported prevalence of adrenal adenomas in an autopsy series is even more variable (1-32%) (4, 5). Most incidentalomas are benign and nonhypersecretory in patients without a known extraadrenal malignancy (3, 6). Some studies have shown that the majority of adrenal masses are benign adenomas, which constitute a significant fraction even in patients with a known extraadrenal primary malignancy (7).
Therefore, distinguishing adenomas from non-adenomatous adrenal masses, particularly metastatic ones, is of great importance in cancer patients. The application of a percutaneous biopsy, which is the gold standard for characterizing adrenal masses, is a suboptimal choice, as it is an invasive procedure with well-documented risks of complications. Furthermore, its accuracy is only 80-90%, due to sampling errors or inadequate specimen sizes in practice(8), and it is least helpful in distinguishing between benign adrenal adenomas and primary adrenocortical carcinomas(9, 10). Noninvasive characterization of masses such as adenomas, is generally preferred (11) because they have the potential to reduce the number of percutaneous biopsies if the results are sufficiently specific (12).
Although most small adrenal masses represent benign cortical adenomas, the morphological features alone cannot reliably differentiate between an adenoma and a primary or metastatic lesion (11, 13). Many studies have suggested that the attenuation value in unenhanced CT, which is expressed in Hounsfield Units (HU), can be used to distinguish between an adenoma and other abnormalities(14-17). The utility of unenhanced CT is based on intracytoplasmic fat, which is often more abundant in adrenal adenomas but rare in adrenal metastases, pheochromocytomas, or adrenocortical carcinomas (18). The threshold values for noncontrast CT in HU units, ranging from 0 HU to 20 HU, have been suggested in the literature, and a value of 10 HU was recommended by a consensus panel organized by the National Institutes of Health (1, 19-24). In practice, we frequently encountered adrenal adenomas with attenuation values > 10 HU. Therefore, many adrenal adenomas will be excluded when applying a threshold of 10 HU on an absolute scale, which would lead to a relatively low sensitivity in differentiating adenomas from nonadenomas and more false negative results. We hypothesized that an attenuation value > 10 HU can be used to differentiate an adenoma from nonadenomas on unenhanced CT. The aim of this study was to determine the optimal threshold of the attenuation values in unenhanced CT for making a distinction between adrenal adenomas and nonadenomas.
One hundred fifteen adrenalectomies were performed at our institution from January 2001 through to July, 2005. The abdominal CT results of all of these cases were reviewed. Seventy cases were excluded from the study for the following reasons: the CT results were unreadable, lost or CT scans were performed with intravenous contrast enhancement but without an unenhanced scan. The other reasons for patient exclusion included a mass sizes < 1 cm in nine patients, the presence of artifacts on the images that prevented the region of interest (ROI) from being measured, and five cases of masses with grossly visible fatty components < -30 HU, which were presumed to be myelolipomas. This left a total 45 cases of adrenal masses, with CT scans containing the available precontrast images, which were reviewed retrospectively. For the five cases with bilateral adrenal masses, the larger of the two masses was selected. There were 17 men and 28 women with a mean age of 46.4 ± 2.2 years (range, 15-72 years). The final diagnosis of these adrenal masses was made by a histopathology examination of the adrenalectomy specimens. The adrenal masses consisted of 25 cortical adenomas and 20 nonadenomas, which in turn consisted of 12 pheochromocytomas, three lymphomas, and five adrenal metastases. The primary malignancies in the cases where the adrenal metastasis was a later development were renal cancers in two patients and one each with ovarian cancer, hepatocellular carcinoma, and breast cancer.
A Somatom Sensation 16 MDCT scanner (Siemens, Forchheim, Germany) was used in 24 patients, with the following settings: 16 × 0.75 mm collimation and 0.5 sec rotation speed, 120 kV, 100-160 mAs, and a 12 mm/rotation table-feed. The final images were reconstructed with a 2 mm increment and a 2 mm thickness. A Light Speed Plus 4 multidetector-row CT(MDCT) scanner (GE Medical Systems, Milwaukee, WI) was used for 13 patients, with the following settings: 120 kV, 240-280 mAs, a table speed of 7.5 mm per rotation, high-quality mode, and a pitch of 3:1. The final scans were reconstructed at 3-mm intervals, with a 2-3 mm slice thickness. A High Speed single-section spiral CT (GE Medical Systems, Milwaukee, WI) was used for the remaining eight patients, with the following settings: 120kV and mA from 200 to 280. The final scans were reconstructed at 3-mm intervals, with a 2-3 mm slice thickness. Twenty-three of the 45 cases followed the adrenal protocol with relatively thin reconstruction intervals and thin slices in each CT machines, while the remaining 22 cases followed the abdomen protocol, abdomen-pelvis protocol, or liver dynamic protocol.
The images were reviewed retrospectively by a single radiologist on a PACS (picture archiving and communication system) workstation (Centricity workstation, version 2.0; GE Medical System). The attenuation of the lesions was measured, using an circular or ovoid ROI set as large as possible but entirely contained within the boundary of the adrenal lesion in order to avoid partial volume averaging with the surrounding tissue. The mass boundary was avoided and areas of necrosis or cystic changes were excluded from the ROI measurement. The range of ROI measurements for adrenal lesions in the CT scans were relatively wide, from 10 mm2 to 776 mm2. The ROI range for adenomas was from 10 mm2 to 597 mm2 and from 20 mm2 to 776 mm2 for nonadenomas. The analysis of CT images was confined to the attenuation value on the unenhanced CT images for the purposes of this study. The other imaging characteristics, such as the attenuation value on the contrast enhancement images, shape, morphology of lesions, weight, or the biochemical state of the patients were not examined. A primary investigator measured the maximum diameter of the masses in the transverse images.
The mean unenhanced attenuation value of the adrenal adenomas and nonadenomas were calculated for each group and analyzed using an unpaired Student's t test, using SPSS software (SPSS for windows, 12.0.0 standard version, LEAD Technologies, Inc.). A p-value < 0.05 was considered significant.
The sensitivity, specificity, positive predictive value, negative predictive value and accuracy for any specific threshold were calculated and compared at 10 HU, 20 HU, and 25 HU. In this study, the sensitivity was defined as the probability that an adrenal mass would be classified as an adenoma, given that it was truly an adenoma. The specificity was defined as the probability that an adrenal mass would be classified as a nonadenoma, given that it is was truly a nonadenoma. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, which were calculated at the cutoff threshold diameters of 2-6 cm, were compared.
The receiver operating characteristic (ROC) curves for the attenuation values at the unenhanced CT and for the mass sizes were generated from the data using SPSS software (SPSS for windows, 12.0.0 standard version, LEAD Technologies, Inc.). For each discriminator, the ROC curve is a plot of the true-positive fraction (TPF = sensitivity) against the false-positive fraction (FPF = 1-specificity). The SPSS program fits the ROC curves to the data, estimates the area under curve as a measure of its effectiveness of a discriminator, and provides the likelihood that the observed differences could have arisen by chance.
The attenuation values on the unenhanced CT ranged from -13.6 to 38.7 HU (mean ± SD; 16.3 ± 1.5) for adenomas and 20.6 to 49.1 HU (38.1 ± 6.8) for nonadenomas. The difference was statistically significant (p = 0.001).
Figure 1 shows a scattergram of the attenuation values on the unenhanced CT of 25 adenomas and each group of 20 nonadenomas. The attenuation values were negative in four (16%) adenomas, > 10 HU in 16 (64%) adenomas, and > 30 HU in seven (28%) adenomas. These seven attenuation values > 30 HU overlapped with the range of nonadenomas. All masses with attenuation < 20 HU were adenomas. Among the 20 nonadenomas, none had an attenuation value < 20. Eighteen (90%) nonadenomas had attenuation values > 30 HU. The attenuation values for the pheochromocytomas ranged from 36.6 HU to 49.1 HU, with a mean value of 40.0 ± 5.7 HU. All the metastases had values > 30 HU ranging from 33.4 HU to 36.6 HU with a mean value of 35.3 ± 1.3 HU. The attenuation values of the three lymphomas were the lowest among the nonadenoma groups, ranging from 20.6 HU to 32.7 HU with a mean value of 25.6 ± 6.1 HU. Table 1 shows the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy calculated for each threshold value. At a threshold value of 10 HU, the sensitivity (36%) and NPV (56%) values were lowest, even though the specificity and PPV were 100%. At a threshold value of 20 HU, the sensitivity and NPV were increased to 60%, while the specificity and positive predictive value were maintained at 100%. At a threshold value of 25 HU, the sensitivity was further increased to 72%, but the specificity (95%) and positive predictive value (95%) decreased.
The mean diameter of the adrenal adenomas was 2.4 ± 1.2 cm ranging from 1.0 cm to 6.3 cm. The mean diameter of the nonadenomas was 5.1 ± 2.4 cm, ranging from 1.0 cm to 9.8 cm. Figure 2 shows a scattergram of the adenomas and nonadenomas according to their size. There were statistically significant differences between the diameter of the adenomas and nonadenomas (p = 0.015). One adrenal metastasis measured 1 cm in the largest diameter, and there were three malignant adrenal masses < 3 cm in the largest diameter. Fourteen (70%) nonadenomas measured < 6 cm in the largest diameter, and all were malignant. Table 2 shows the sensitivity, specificity, PPV, NPV, and accuracy at each tumor diameter. In ROC analysis, the area under the ROC curve for CT attenuation values (0.904 ± 0.044) was larger than that for size (0.860 ± 0.060) (Fig. 3), which indicates the unenhanced attenuation value is more useful in distinguishing between adenomas and nonadenomas than the mass size (Figs. 4, 5, 6).
The high lipid content of most adrenal cortical adenomas produces the low attenuation values on the CT images(25). Significant differences in the mean lipid content have been documented between benign adrenal adenomas and adrenal carcinomas both in vitro and in vivo (26). The mean lipid content in adrenal adenomas was 13.4%, compared with 3.5% in adrenal carcinomas, which is a distinctive feature that can be used to differentiate adenomas from other adrenal masses (27). Some researchers have reported negative mean attenuation values for adrenal adenomas, with a significant difference in mean attenuation values between adenomas and nonadenomas. A few reports found positive mean attenuation values for adrenal adenomas in nonenhanced CT, ranging from 2.2 to 13.0 HU (15, 17, 28). In the present study, the 25 adrenal adenomas had a mean attenuation value of 16.3 ± 14.9, which is higher than those reported elsewhere. The unenhanced CT has proven to be a useful tool for making a radiological diagnosis of adrenal cortical adenomas (18). Several studies have attempted to define a specific threshold to distinguish between benign and malignant lesions, and many studies suggested a cutoff threshold of between 0 and 18 HU as the optimal value (12, 15, 17, 21, 28). Using a lower threshold value tends to produce a high specificity for lesion characterization but poor sensitivity, which results in many adrenal lesions requiring additional CT or MR imaging. On the other hand, threshold values toward the high end of the spectrum tend to produce high sensitivity but poor specificity for tissue characterization.
The specificity for making a diagnosis of adrenal adenoma needs to be close to 100% in order to prevent misdiagnoses of adrenal metastasis. However, the sensitivity is also important for practical reasons because of the high incidence of adenoma, compared with that of a nonadenoma. In order to prevent missing malignancies, applying low threshold values with a high specificity and low sensitivity will result in more indeterminate lesions, which will necessitate further imaging or a percutaneous biopsy. Boland et al. (21) proposed that higher threshold values for identifying adrenal lesions may be a better choice, depending on the relative incidence of benign and malignant lesions, as well as the costs and benefits of avoiding false negative or false positive diagnoses. In this study, a threshold value of 20 HU provided a specificity, sensitivity, PPV and accuracy of 100%, 60%, 100%, and 56%, respectively. At a threshold value of 25 HU, the specificity decreased to 95%, but the sensitivity and accuracy increased to 72% and 82%, respectively. Based on these results, the optimal threshold CT attenuation value for differentiating between an adrenal adenoma and nonadenoma is 20 HU or higher, which is higher than the values recommended in previous studies.
Recently many reports have suggested that the percentage change in the washout of the contrast material on the dynamic and delayed contrast-enhanced CT can be a highly specific method for differentiating adrenal adenomas from nonadenomas (22, 29). These suggestions are based on the observation that adrenal adenomas show more rapid and greater washout of the contrast material than adrenal nonadenomas. Therefore, the percentage change in washout is a useful criterion for differentiating adrenal adenomas from nonadenomas (29, 30). This method is particularly important for lipid-poor adenomas because there is significant overlap of the mean attenuation values on unenhanced CT between the lipid-poor adrenal adenomas and nonadenomas (30). Caoili et al. (30) suggested that almost all adrenal masses can be correctly categorized as adenomas or nonadenomas with a combination of unenhanced and delayed enhanced CT. In this study, the adrenal adenomas were not classified into the lipid-rich and lipid poor types, and there was some overlap of the unenhanced attenuation values over 30 HU between the adenomas and nonadenomas. Moreover, the unenhanced CT attenuation value on its own does not allow accurate differentiation in this overlapped groups. In indeterminate adrenal lesions in unenhanced CT, particularly in patients with a history of a malignancy, additional work-up will be needed to diagnose and differentiate adenomas from nonadenomas by calculating the washout profile of contrast in the enhanced CT. Regardless of some overlap in the range of attenuation values > 30 HU on the unenhanced CT between two groups, which will require additional work up, the results of this study suggest that attenuation value 20 HU or 25 HU may be an acceptable cut off value on unenhanced CT scan to differentiate between adrenal adenomas and nonadenomas with a high specificity and sensitivity.
Many studies have suggested that the size of adrenal tumors is an important determinant for differentiating adrenal adenomas from nonadenomas (31-33). There is a significant increase in the frequency of malignancy with increasing adrenal tumor size (32, 33). Korobkin et al. (34), in a study of 135 adrenal masses, reported that the mean diameter of adenomas was significantly lower than that of nonadenomas (2.4 versus 4.5 cm) but that there was a sufficient overlap between the two groups, particularly with the smaller lesions. These results showed very similar results, with the mean maximal diameter of adenomas being significantly smaller than that of nonadenomas. Grouping the adrenal masses by their size at 4 cm, 5 cm, and 6 cm in the largest diameter yielded a relatively high sensitivity of more than 90% but a specificity of only 25%-70%. The group with masses 2 or 3 cm in the largest diameter showed increased specificity but decreased sensitivity. Therefore, these results support previous reports in that the size criteria are of little value when discriminating between benign adrenal masses and adrenal gland metastasis (6, 35).
This study had several limitations. First, there was a relatively small number of cases, with a particularly small number of metastases included in the nonadenomas group. In a literature review, Hamrahian et al. (36) performed their study, based on 157 adrenal adenomas/hyperplasia and 142 nonadenomas, including 35 adrenal metastases. Miyake et al. analyzed 14 nonfunctioning adrenal adenomas and 22 nonadenomas, including 16 metastases (19). Korobkin et al. examined 93 cortical adrenal adenomas and 42 malignant adrenal lesions, including 34 metastases (12). However, not all of the cases included in those studies have been verified pathologically. The second limitation is that several CT machines were used, and the scan parameters were not standardized and there was a relatively wide range of mAs, from 100-160 and 200-280 mAs. However, the study population included a long clinical follow-up and the results obtained from those diverse CT systems may suggest the general applicability of these results to future cases. Thirdly, there is a possibility of selection bias in the group of adrenal adenomas. Adrenal lesions with 10 HU or less on the unenhanced CT usually undergo a CT follow up rather than an adrenalectomy or do not undergo additional follow up. In this study, all data regarding adenomas were based on pathologically confirmed ones by an adrenalectomy only. Adenomas diagnosed with an additional CT scan without a pathological diagnosis were excluded.
In conclusion, 20 or 25 HU is proposed as the optimal threshold attenuation value on the nonenhanced CT for discriminating between adrenal adenomas and nonadenomas. These results show that the attenuation values on the unenhanced CT are superior to the size criteria in differentiating adrenal adenomas from an adrenal malignancy, and grouping by the tumor size is of little value due to the low specificity and sensitivity.
Figures and Tables
Fig. 1
Scattergram of the attenuation values in the unenhanced CT of 25 adenomas and each group of 20 nonadenomas (NA-L: lymphoma, NA-M: metastasis, NA-P: pheochromocytoma). Note that all masses with a HU < 20 were adenomas. Among the 20 nonadenomas, none had an attenuation value < 20, and eighteen (90%) of the nonadenomas had attenuation values > 30 HU.

Fig. 3
Receiver operating characteristic curve for unenhanced CT attenuation value in enabling differentiation of adenomas from nonadenomas (A) and receiver operating characteristic curve for the size of adrenal lesions (B). The area under the receiver operating characteristic curve for CT attenuation values (0.904±0.044) was larger than that for size (0.860±0.060), indicating a greater ability of unenhanced CT attenuation value for distinguishing adenomas from nonadenomas.

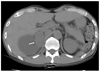
Fig. 4
Example of a typical adrenal adenoma in the noncontrast image. A 1.6 × 1.2 cm, well-defined, low attenuated nodule (arrow) is shown in the left adrenal gland with a HU value of -13.6. This nodule was confirmed to be an adrenal adenoma by a pathological examination.
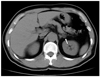
Fig. 5
Non-contrast CT image of a 57 year-old female adrenal gland, showing a 2.9 × 2.7 cm, round-shaped nodule (arrow) in the left adrenal gland with a HU value of 38.7. The nodule was confirmed to be a benign adrenal adenoma by a pathological examination.
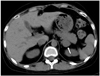
Fig. 6
Noncontrast abdominal CT scan of 17-year-old male, showing a 5.4 × 3.3 cm right adrenal mass (arrow) with a HU value of 33.4, which was suggestive of a nonadenoma rather than an adrenal adenoma. This mass was confirmed by a pathological examination to be a right adrenal metastasis from a renal cell carcinoma.
References
1. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995. 16:460–484.
2. Caplan RH, Strutt PJ, Wickus GG. Subclinical hormone secretion by incidentally discovered adrenal masses. Arch Surg. 1994. 129:291–296.
3. Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991. 110:1014–1021.
4. Kokko JP, Brown TC, Berman MM. Adrenal adenoma and hypertension. Lancet. 1967. 1:468–470.
5. Dobbie JW. Adrenocortical nodular hyperplasia: the ageing adrenal. J Pathol. 1969. 99:1–18.
6. Abecassis M, McLoughlin MJ, Langer B, Kudlow JE. Serendipitous adrenal masses: prevalence, significance, and management. Am J Surg. 1985. 149:783–788.
7. Oliver TW, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984. 153:217–218.
8. Welch TJ, Sheedy PF 2nd, Stephens DH, Johnson CM, Swensen SJ. Percutaneous adrenal biopsy: review of a 10-year experience. Radiology. 1994. 193:341–344.
9. Heaston DK, Handel DB, Ashton PR, Korobkin M. Narrow gauge needle aspiration of solid adrenal masses. AJR Am J Roentgenol. 1982. 138:1143–1148.
10. Tang CK, Gray GF. Adrenocortical neoplasms. Prognosis and morphology. Urology. 1975. 5:691–695.
11. Bernardino ME, Walther MM, Phillips VM, Graham SD Jr, Sewell CW, Gedgaudas-McClees K, et al. CT-guided adrenal biopsy: accuracy, safety, and indications. AJR Am J Roentgenol. 1985. 144:67–69.
12. Korobkin M, Brodeur FJ, Yutzy GG, Francis IR, Quint LE, Dunnick NR, et al. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR Am J Roentgenol. 1996. 166:531–536.
13. Glazer HS, Weyman PJ, Sagel SS, Levitt RG, McClennan BL. Nonfunctioning adrenal masses: incidental discovery on computed tomography. AJR Am J Roentgenol. 1982. 139:81–85.
14. Miyake H, Maeda H, Tashiro M, Suzuki K, Nagatomo H, Aikawa H, et al. CT of adrenal tumors: frequency and clinical significance of low-attenuation lesions. AJR Am J Roentgenol. 1989. 152:1005–1007.
15. Lee MJ, Hahn PF, Papanicolaou N, Egglin TK, Saini S, Mueller PR, et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology. 1991. 179:415–418.
16. van Erkel AR, van Gils AP, Lequin M, Kruitwagen C, Bloem JL, Falke TH. CT and MR distinction of adenomas and nonadenomas of the adrenal gland. J Comput Assist Tomogr. 1994. 18:432–438.
17. Singer AA, Obuchowski NA, Einstein DM, Paushter DM. Metastasis or adenoma? Computed tomographic evaluation of the adrenal mass. Cleve Clin J Med. 1994. 61:200–205.
18. Korobkin M, Giordano TJ, Brodeur FJ, Francis IR, Siegelman ES, Quint LE, et al. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996. 200:743–747.
19. Miyake H, Takaki H, Matsumoto S, Yoshida S, Maeda T, Mori H. Adrenal nonhyperfunctioning adenoma and nonadenoma: CT attenuation value as discriminative index. Abdom Imaging. 1995. 20:559–562.
20. McNicholas MM, Lee MJ, Mayo-Smith WW, Hahn PF, Boland GW, Mueller PR. An imaging algorithm for the differential diagnosis of adrenal adenomas and metastases. AJR Am J Roentgenol. 1995. 165:1453–1459.
21. Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol. 1998. 171:201–204.
22. Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. AJR Am J Roentgenol. 1998. 170:747–752.
23. Bertherat J, Mosnier-Pudar H, Bertagna X. Adrenal incidentalomas. Curr Opin Oncol. 2002. 14:58–63.
24. Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass ("incidentaloma"). Ann Intern Med. 2003. 138:424–429.
25. Nwariaku FE, Champine J, Kim LT, Burkey S, O'Keefe G, Snyder WH. Radiologic characterization of adrenal masses: the role of computed tomography-derived attenuation values. Surgery. 2001. 130:1068–1071.
26. Leroy-Willig A, Roucayrol JC, Luton JP, Courtieu J, Niesenbaum N, Louvel A. In vitro adrenal cortex lesions characterization by NMR spectroscopy. Magn Reson Imaging. 1987. 5:339–344.
27. Leroy-Willig A, Bittoun J, Luton JP, Louvel A, Lefevre JE, Bonnin A, et al. In vivo MR spectroscopic imaging of the adrenal glands: distinction between adenomas and carcinomas larger than 15 mm based on lipid content. AJR Am J Roentgenol. 1989. 153:771–773.
28. Päivänsalo M, Lähde S, Merikanto J, Kallionen M. Computed tomography in primary and secondary adrenal tumours. Acta Radiol. 1988. 29:519–522.
29. Szolar DH, Kammerhuber FH. Adrenal adenomas and nonadenomas: assessment of washout at delayed contrast-enhanced CT. Radiology. 1998. 207:369–375.
30. Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002. 222:629–633.
31. Mayo-Smith WW, Boland GW, Noto RB, Lee MJ. State-of-the-art adrenal imaging. Radiographics. 2001. 21:995–1012.
32. Hussain S, Belldegrun A, Seltzer SE, Richie JP, Gittes RF, Abrams HL. Differentiation of malignant from benign adrenal masses: predictive indices on computed tomography. AJR Am J Roentgenol. 1985. 144:61–65.
33. Copeland PM. The incidentally discovered adrenal mass. Ann Intern Med. 1983. 98:940–945.
34. Korobkin M, Francis IR, Kloos RT, Dunnick NR. The incidental adrenal mass. Radiol Clin North Am. 1996. 34:1037–1054.
35. Mitnick JS, Bosniak MA, Megibow AJ, Naidich DP. Nonfunctioning adrenal adenomas discovered incidentally on computed tomography. Radiology. 1983. 148:495–499.
36. Hamrahian AH, Ioachimescu AG, Remer EM, Motta-Ramirez G, Bogabathina H, Levin HS, et al. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab. 2005. 90:871–877.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download