Abstract
Objective
The aim of the study was to compare the accuracy of magnetic resonance imaging (MRI) and mammography for the detection and assessment of the size of ductal carcinoma in situ (DCIS).
Materials and Methods
The preoperative contrast-enhanced MRI and mammography were analyzed in respect of the detection and assessment of the size of DCIS in 72 patients (age range: 30-67 years, mean age: 47 years). The MRI and mammographic measurements were compared with the histopathologic size with using the Pearson's correlation coefficients and the Mann-Whitney u test. We evaluated whether the breast density, the tumor nuclear grade, the presence of comedo necrosis and microinvasion influenced the MRI and mammographic size estimates by using the chi-square test.
Results
Of the 72 DCIS lesions, 68 (94%) were detected by MRI and 62 (86%) were detected by mammography. Overall, the Pearson's correlation of the size between MRI and histopathology was 0.786 versus 0.633 between mammography and histopathology (p < 0.001). MRI underestimated the size by more than 1 cm (including false negative examination) in 12 patients (17%), was accurate in 52 patients (72%) and overestimated the size by more than 1 cm in eight patients (11%) whereas mammography underestimated the size in 25 patients (35%), was accurate in 31 patients (43%) and overestimated the size in 16 patients (22%). The MRI, but not the mammography, showed significant correlation for the assessment of the size of tumor in noncomedo DCIS (p < 0.001 vs p = 0.060). The assessment of tumor size by MRI was affected by the nuclear grade (p = 0.008) and the presence of comedo necrosis (p = 0.029), but not by the breast density (p = 0.747) or microinvasion (p = 0.093).
Ductal carcinoma in situ (DCIS) accounts for 15-20% of all detected breast cancers and for 25-56% of the clinically occult cancers detected by mammography (1). On mammography, from 62 to 98% of DCIS lesions are detected by the presence of calcifications, with from 2 to 23% of them manifesting only as a mass or an asymmetric density. DCIS is a multiform disease with different growth patterns and heterogeneous set of clinical signs and symptoms (1-3).
Conservative surgery of breast cancer has recently become an increasingly common treatment for DCIS (4). Accurate information about the tumor size and its distribution is important in preoperative treatment planning. Mammography, however, has relative limitations for detecting DCIS and assessing the tumor size because noncalcified DCIS foci are not depictable in the dense breasts, and calcifications associated with benign histology are sometimes difficult to differentiate from malignant calcifications. Recent studies have shown that magnetic resonance imaging (MRI) is more accurate than mammography for detecting and assessing the tumor size in patients with invasive cancer (5-10). However, the diagnostic value of MRI for detecting and predicting the tumor size of DCIS is still controversial (11-14).
The purpose of this study was to compare the accuracy of MRI and mammography for the detection and assessment of the size of DCIS.
Between October 2003 and September 2005, 260 patients were diagnosed with DCIS at our department. Of these patients, 100 patients had breast MRI performed before surgery. Cases (n = 19) that underwent excisional biopsy before MRI and multifocal or multicentric lesions (n = 9) were excluded from the study, because of the difficulty in estimating the exact tumor size. Thus, 72 patients (age range: 30-67 years, mean age: 47 years) were included in this study. Of these 72 patients, 43 (60%) presented with a screening mammographic abnormality, 22 (31%) with a palpable mass, six (8%) with breast pain and one (1%) with bloody nipple discharge. Forty patients underwent breast conservative operations, and 32 patients underwent mastectomy. This study was conducted with the approval of our institutional review board; informed consent was not required.
Mammography was performed with a full-field digital mammography system (Senographe 2000D, GE Medical Systems, Milwaukee, WI). Routine mediolateral oblique and craniocaudal mammograms were obtained in all the patients, and additional spot-compression with magnification and true lateral images were available in all but one patient. Of the 72 patients, five (7%) had almost entirely fatty breasts (BI-RADS grade 1, < 25% glandular), ten (14%) had scattered fibroglandular tissue in fatty breasts (grade 2, approximately 25-50% glandular), 33 (46%) had heterogeneously dense breasts (grade 3, approximately 51-75% glandular) and 24 (33%) had extremely dense breasts (grade 4, > 75% glandular). On the mammography, the index lesion was seen as microcalcifications alone in 31 (43%) patients and as a mass with (n = 4) or without (n = 27) microcalcifications in 31 (44%) patients. No mammographic abnormalities were found in ten (14%) patients.
MRI was performed with the patient in a prone position and with using a dedicated phase-array breast coil. Of the 72 patients, a 1.5-T system (Magnetom Sonata, Siemens Medical Solutions, Erlangen, Germany) was used in 66 patients. Unenhanced fat-suppressed T2-weighted turbo spin echo sagittal images were obtained. The image parameters were TR/TE = 9120/82, flip angle: 180 degree and field of view (FOV): 170 ×170 mm. The slice thickness was 1.0 mm. Gadolinium-DTPA (Magnevist [0.1 mmol/kg], Schering, Berlin, Germany) was administrated manually as an intravenous injection. T1 three-dimensional fast low angle shot (3D FLASH) dynamic sequences were performed with one pre-enhanced and four post-enhanced series in the unilateral sagittal images. The image parameters were TR/TE = 4.9/1.8, flip angle: 0 degree and FOV: 170 ×170 mm. The acquisition time was 84 seconds, and the slice thickness was 1-1.6 mm without a gap. The interval between the start of the image acquisition and the contrast material injection was 15 seconds. The post processing included early subtraction (i.e., first post-contrast images minus pre-contrast images) and reverse subtraction (i.e., first post-contrast images minus fourth post-contrast images) for the dynamic studies, the calculation of the time-intensity curves of the enhancing regions and the maximum intensity projection. After the dynamic studies, delayed T1 weighted spin echo images were acquired in the axial planes (TR/TE: 718/11, flip angle: 90 degrees, 2 mm thickness, 3.2 mm gap, FOV: 300 ×300 mm). The approximate total time was 30 minutes per one examination. A 1.5 T imager (Signa; GE Medical Systems, Milwaukee, WI) was used in six patients. Unenhanced fat-suppressed T2-weighted turbo spin echo sagittal images were obtained. The image parameters were TR/TE = 5000/105, flip angle: 90 degrees and FOV: 170 ×170 mm. The slice thickness was 1.0-5.0 mm. Gadolinium-DTPA (Magnevist [0.1 mmol/kg], Schering, Berlin, Germany) was administrated manually as an intravenous injection. A T1- three dimensional spoiled gradient-echo (SPGR) sequence in the unilateral sagittal images was performed, with one pre-contrast and two post-contrast series. The image parameters were TR/TE = 19.7/1.8, flip angle: 0 degree and FOV: 170 ×170 mm. The acquisition time was 240 seconds and the slice thickness was 1.0-2.5 mm without a gap. MRI and mammography were performed on the same day.
The diagnosis of DCIS was obtained before the preoperative MRI and mammography by fine needle aspiration in 13 patients and 14-guage (n = 18) or 11-guage (n = 34) core biopsy in 52 patients. Frozen biopsy was performed during the operation in the remaining seven patients. All the patients underwent surgery within one week of the preoperative MRI and mammography. In our institution, most patients with a tumor larger than 5 cm and the patients with multicentric cancer undergo mastectomy instead of a breast conservative operation. The patient's preference and the size of the breast were considered in all the cases.
After lumpectomy or mastectomy, a gross specimen was evaluated with serial 10 mm slices by the pathologists, and additional slices were prepared from any gross suspicious areas. The maximum lesion size by histopathology was used as a reference standard. DCIS was classified according to the nuclear grade (high and non-high), the presence of comedo necrosis (comedo and noncomedo type) and microinvasion (microinvasive and pure). Non-high nuclear grade included the low and intermediate grade DCIS. We defined microinvasion as an extension of the cancer cells beyond the basement membrane into the adjacent tissue with no focus, more than 0.1 cm in diameter.
All lesions detected by MRI and mammography were retrospectively analyzed and assessed in consensus by two radiologists. At the time of the retrospective review, the radiologists were not aware of the histopathologic results, clinical information or the other radiologic images. Microcalcifications of pleomorphic shape, linear, ductal or segmental distribution, irregular mass or asymmetry were defined as positive mammography results (1). A positive MRI was defined if the signal intensity was higher than that of the breast parenchyma on the early subtraction images, and the morphology presented as clumped or heterogeneous ductal enhancement or a focal area of enhancement with irregular borders (5, 6, 15). We considered a mass as benign if it was well-circumscribed and homogeneous enhancement on the early subtraction images or high signal intensity on T2-weighted images. The best image depicting the abnormality was selected from the early subtraction images. The tumor size was measured by assessing the longest axis of the lesion with using electronic calipers. When no lesion was present on the MRI or mammography, the size was set to 0 cm.
After analyzing the MRI and mammography, the pathologic and surgical records were reviewed by one of the two radiologists. The accuracy of the assessment of the tumor size by MRI and mammography was evaluated, and the cases were classified as accurate, underestimation or overestimation. Accurate estimation was defined when the difference between the imaging and histopathologic size of the lesion was less than 1 cm, underestimation was defined as no visualization or cases that were underestimated by more than 1 cm with an imaging modality, and overestimation was defined as cases overestimated by more than 1 cm with an imaging modality as compared with the histopathologic size. If there was a change in the operative methods after MRI, then it was recorded.
Pearson's correlation coefficients were calculated to determine the association between the MRI and mammographic measurements and the histopathologic size. Correlation coefficients were calculated between the MRI or mammographic measurement and the histopathologic size, according to the breast density, the mammographic findings (either microcalcifications alone or a mass with or without microcalcifications), the nuclear grade, the presence of comedo necrosis and microinvasion. Graphs were used to present the relationship between the MRI and mammographic measurements and the histopathologic size. The Mann-Whitney u test was used to evaluate the statistical significance of the differences in size between MRI or mammography and the histopathology. We also evaluated whether the breast density, the mammographic findings, the tumor nuclear grade and the presence of comedo necrosis and microinvasion influenced the assessment of the size of DCIS by MRI and mammography by using the chi-square test. A p value of less than 0.05 was considered statistically significant.
The histopathologic examinations revealed high grade DCIS in 43 patients and non-high grade DCIS in 29 patients. The comedo type was noted in 40 patients and the noncomedo type was noted in 32 patients. Pure DCIS was found in 58 patients and DCIS with microinvasion was found in 14. Forty lesions were located in the left breast, and 32 were located in the right. Of the 72 DCIS lesions, eight were detected only by MRI, 60 by MRI and mammography and two only by mammography. Two DCIS lesions (3 cm non-high grade, noncomedo DCIS and 0.7 cm high grade, comedo DCIS) were detected only by pathology. Thus, 68 (94%) were detected by MRI and 62 (86%) were detected by mammography. All the 10 false negative lesions detected by mammography were within the dense pattern of breast parenchyma, but seven of these lesions were detected by MRI. For the four false negative lesions detected by MRI, two were detected as microcalcifications by mammography. Three of these four false negative lesions by MRI were the non-high grade, noncomedo type, and one was the high-grade, comedo type. The mean histopathologic size of the false negative lesions by mammography was 2.3 cm (0.7-4.5 cm) and that by MRI was 2.1 cm (0.5-4.0 cm), whereas the mean tumor size detected by mammography was 3.2 cm (0.4-10.0 cm) and that by MRI was 3.1 cm (0.4-10.0 cm), which revealed no significant statistical differences.
The overall mean size as predicted by MRI and mammography was 3.2 cm (0-10.0 cm) and 2.7 cm (0-11.2 cm), respectively, compared with 3.1 cm (0.4-10.0 cm) on the histopathology (Table 1). The overall Pearson's correlation coefficient for the size between MRI and histopathology was 0.786 (p < 0.001) and that for the size between mammography and histopathology was 0.633 (p < 0.001). On MRI, the size correlation coefficients of the high grade, comedo type and the DCIS with microinvasion were higher than those of the non-high grade, noncomedo type and pure DCIS (Table 1). MRI, but not mammography, showed a significant correlation with the actual tumor size for noncomedo DCIS (p < 0.001 vs p = 0.060). The graph depicted a close fit between the MRI and histopathologic sizes (Fig. 1). The size discrepancy and the span were smaller for the MRI measurements, as compared with the mammographic differences. The mean absolute difference in the size for all lesions was 0.8 cm (0-6.5 cm) on MRI and 1.5 cm (0-7.3 cm) on mammography.
MRI underestimated the size of DCIS in 12 patients (17%), was accurate in 52 patients (72%) and overestimated the size in eight patients (11%) (Figs. 2, 3, 4), whereas mammography underestimated the size of DCIS in 25 patients (35%), was accurate in 31 patients (43%) and overestimated the size in 16 patients (22%) (Table 2). MRI was more accurate than mammography in assessing the lesion size (p < 0.001, Mann-Whitney u test). Based on these MRI findings, a change of the operative methods was found in 13 patients (18%). A change of the planned mastectomy to necessary breast conservative operation was in five patients (7%), and that of the planned breast conservative operation to necessary mastectomy was in six patients (8%). However, two patients (3%) underwent unnecessary mastectomy instead of the planned breast conservative operation.
Of the 72 patients who were included into the study, accurate assessment of the size of the lesion by mammography was found in 47% (seven of 15) with fatty breasts (BI-RADS grade 1 or 2 breast density) and 42% (24 of 57) with dense breasts (BI-RADS grade 3 or 4 breast density) (p = 0.751), 65% (20 of 31) with microcalcifications alone and 35% (11 of 31) with a mass with or without microcalcifications (p = 0.004), 49% (21 of 43) with high grade DCIS and 34% (10 of 29) with non-high grade DCIS (p = 0.228), 45% (18 of 40) with comedo DCIS and 41% (13 of 32) with noncomedo DCIS (p = 0.709), 57% (8 of 14) with microinvasive DCIS and 40% (23 of 58) with pure DCIS (p = 0.236), whereas accurate assessment of the size by MRI was found in 67% (ten of 15) with fatty breasts and 74% (42 of 57) with dense breasts (p = 0.747), 77% (24 of 31) with microcalcifications alone and 77% (24 of 31) with a mass with or without microcalcifications (p = 0.999), 84% (36 of 43) with high grade DCIS and 55% (16 of 29) with non-high grade DCIS (p = 0.008), 83% (33 of 40) with comedo DCIS and 59% (19 of 32) with noncomedo DCIS (p = 0.029), 93% (13 of 14) with microinvasive DCIS and 67% (39 of 58) with pure DCIS (p = 0.093) (Table 2).
The presence of dense tissue on mammography often obscures the tumor, and this makes detection and size assessment difficult (11). In our study, all the 10 false negative lesions detected by mammography were in patients with dense breasts. However, the detection of DCIS and the assessment of the size by MRI were not significantly affected by the breast density. The assessment of the tumor size by MRI was accurate (within 1 cm as compared with the histopathologic size) in 72% (52 of 72), whereas the mammographic assessment was accurate in 43% (31 of 72). As compared with mammography, MRI showed significant correlation in the assessment of the tumor size in both the noncomedo (p < 0.001 vs p = 0.06) and comedo DCIS (p < 0.001 vs p < 0.001). Thus, our results suggest that MRI has the potential to provide more information for preoperative planning particularly in patients with dense breasts and with noncomedo DCIS, as compared with mammography.
The previously reported sensitivities for detecting DCIS lesions by MRI have varied; they have ranged from 40% to 100%, which can possibly be explained by the variable levels of angiogenesis in these lesions or the different MRI techniques (5, 6, 12-16). Even if we exclude DCIS with microinvasion, the sensitivity of detecting DCIS lesions by MRI was 93% (54 of 58), and this was better than a recent multicenter trial with the sensitivity of 73% (46 of 63) (17). Several studies have reported that the variable detected rate by MRI may be related to the tumor size (13, 17, 18). In our study, the histopathologic size of the false negative cases by MRI ranged from 0.5 cm to 4.0 cm (mean, 2.1 cm), whereas the size of the detected cases by MRI ranged from 0.4 cm to 10.0 cm (mean, 3.1 cm), and this difference was not statistically significant. Thus the size of the lesions alone was an incomplete explanation for the variable reported detection sensitivities of DCIS by MRI. Of the four DCIS lesions not detected by MRI in our study, three were the non-high grade and noncomedo type. Assessment of the tumor size by MRI was affected by the nuclear grade (p = 0.008) and the presence of comedo necrosis (p = 0.029); accurate estimation of the tumor size was found in 84% (36 of 43 patients) of the high grade DCIS and 83% (33 of 40 patients) of the comedo DCIS, whereas accurate estimation of the tumor size was found in only 55% (16 of 29 patients) of the non-high grade DCIS and 59% (19 of 32 patients) of the noncomedo DCIS. High grade or comedo DCIS tends to be more aggressive, which may explain the early contrast enhancement and the high sensitivity by MRI (19, 20). Our study suggests that histopathologic characteristics of tumor affect the sensitivity of detection of DCIS and the assessment of the tumor size by MRI.
Our study had limitations. First, this study was a retrospective design. Second, multicentric or multifocal cancer was excluded Third, although this study included 72 patients, some of the subgroups contained only few patients, which limited the reliability of the results.
In conclusion, MRI was more accurate for the detection and assessment of the size of DCIS than mammography. As compared with mammography, MRI showed significant correlation for the assessment of the tumor size in noncomedo DCIS, and the assessment of the tumor size was affected by the nuclear grade and the presence of comedo necrosis.
Figures and Tables
Fig. 1
Graph showing the correlation between the histopathologically determined DCIS size and the corresponding DCIS size as measured by MRI and mammography. The mean absolute difference in the size between imaging and histopathology was 0.8 cm (0-6.5 cm) by MRI and 1.5 cm (0-7.3 cm) by mammography. DCIS = Ductal carinoma in situ, MRI = Magnetic resonance imaging, MMG = Mammography.

Fig. 2
A 45-year-old woman with a 5 cm high grade, comedo type DCIS, which was more accurately assessed by MRI than by mammography.
A. Spot-magnification mediolateral oblique mammogram shows a 1.1 cm cluster of the pleomorphic microcalcifications (arrow) in the left breast. The metallic marker is on the palpable area.
B. Dynamic contrast-enhanced sagittal subtraction MR image shows a 5 cm sized, segmentally distributed, heterogeneous enhancing lesion (arrows) in the left breast.
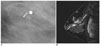
Fig. 3
A 43-year-old woman with a 2 cm high grade, comedo type DCIS, which was more accurately assessed by mammography than by MRI.
A. Spot-magnification mediolateral oblique mammogram shows a 1.4 cm segmental distribution of the pleomorphic microcalcifications (arrow) in the right breast.
B. Dynamic contrast-enhanced sagittal subtraction MR image shows a 0.5 cm sized, irregular enhancing lesion (arrow) in the right breast.
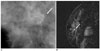
Fig. 4
A 33-year-old woman with a 2.7 cm high grade, comedo type DCIS, which was accurately assessed by both MRI and mammography.
A. Spot-magnification mediolateral oblique mammogram shows a 2.7 cm segmental distribution of the pleomorphic microcalcifications (arrow) in the left breast.
B, C. Dynamic contrast-enhanced sagittal subtraction MR images shows a 2.7 cm sized, clumped ductal enhancement (arrows) in the left breast.
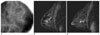
Table 1
Mean Size of DCIS and the Correlation Coefficients by MRI and Mammography According to the Breast Density, the Mammographic Findings and the Histopathologic Results

Table 2
Assessment of the Size of DCIS by MRI and Mammography According to the Breast Density, the Mammographic Findings and the Histopathologic Result

Note.-DCIS = ductal carcinoma in situ
* Accurate estimation was defined as less than 1 cm for the difference between the imaging and histopathologic sizes.
† Underestimation was defined as no visualization or cases underestimated by more than 1 cm with an imaging modality as compared with the histopathologic size.
‡ Overestimation was defined as cases overestimated by more than 1 cm with an imaging modality as compared with the histopathologic size.
§ No mammographic abnormalities were noted in ten (14%) patients.
References
1. Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology. 1989. 170:411–415.
2. Stomper PC, Connolly JL, Meyer JE, Harris JR. Clinically occult ductal carcinoma in situ detected with mammography: analysis of 100 cases with radiologic-pathologic correlation. Radiology. 1989. 172:235–241.
3. Ikeda DM, Andersson I. Ductal carcinoma in situ (DCIS): atypical mammographic appearances. Radiology. 1989. 172:661–666.
4. Silverstein MJ. Ductal carcinoma in situ (DCIS) of the breast: diagnostic and therapeutic controversies. J Am Coll Surg. 2001. 192:196–214.
5. Orel SG, Schnall MD, LiVolsi VA, Troupin RH. Suspicious breast lesions: MR imaging with radiologic-pathologic correlation. Radiology. 1994. 190:485–493.
6. Soderstrom CE, Harms SE, Copit DS, Evans WP, Savino DA, Krakos PA, et al. Three-dimensional RODEO breast MR imaging of lesions containing ductal carcinoma in situ. Radiology. 1996. 201:427–432.
7. Kristoffersen Wiberg M, Aspelin P, Sylvan M, Bone B. Comparison of lesion size estimated by dynamic MR imaging, mammography and histopathology in breast neoplasms. Eur Radiol. 2003. 13:1207–1212.
8. Mumtaz H, Hall-Craggs MA, Davidson T, Walmsley K, Thurell W, Kissin MW, et al. Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol. 1997. 169:417–424.
9. Morris EA, Liberman L, Ballon DJ, Robson M, Abramson AF, Heerdt A, et al. MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003. 181:619–626.
10. Hata T, Takahashi H, Watanabe K, Takahashi K, Taguchi K, Itoh T, et al. Magnetic resonance imaging for preoperative evaluation of breast cancer: a comparative study with mammography and ultrasonography. J Am Coll Surg. 2004. 198:190–197.
11. Van Goethem M, Schelfout K, Dijckmans L, Van Der Auwera JC, Weyler J, Verslegers I, et al. MR mammography in the preoperative staging of breast cancer in patients with dense breast tissue: comparison with mammography and ultrasound. Eur Radiol. 2004. 14:809–816.
12. Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995. 197:743–747.
13. Gilles R, Zafrani B, Guinebretiere JM, Meunier M, Lucidarme O, Tardivon AA, et al. Ductal carcinoma in situ: MR imaging-histopathologic correlation. Radiology. 1995. 196:415–419.
14. Heywang-Kobrunner SH. Contrast-enhanced magnetic resonance imaging of the breast. Invest Radiol. 1994. 29:94–104.
15. American College of Radiology. Breast imaging reporting and data system (BI-RADS). 2003. Reston, Va: American College of Radiology.
16. Boetes C, Strijk SP, Holland R, Barentsz JO, Van Der Sluis RF, Ruijs JH. False-negative MR imaging of malignant breast tumors. Eur Radiol. 1997. 7:1231–1234.
17. Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004. 292:2735–2742.
18. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.
19. Guidi AJ, Fischer L, Harris JR, Schnitt SJ. Microvessel density and distribution in ductal carcinoma in situ of breast. J Natl Cancer Inst. 1994. 86:614–619.
20. Neubauer H, Li M, Kuehne-Heid R, Schneider A, Kaiser WA. High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: characteristic findings for signal increased and morphological pattern of enhancement. Br J Radiol. 2003. 76:3–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download