Abstract
Objective
To assess the feasibility and safety of polyvinyl alcohol (PVA) embolization adjuvant to transarterial oily chemoembolization (P-TACE) in advanced hepatocellular carcinoma (HCC) with arterioportal shunts (APS).
Materials and Methods
Nineteen patients who underwent PVA embolization for APS before a routine chemoembolization (TACE) procedure were retrospectively reviewed. 10 of these 19 patients underwent follow-up TACE or P-TACE after P-TACE (Group A), but nine patients underwent only initial P-TACE because of progression of HCC and/or underlying liver cirrhosis (Group B). Hepatic function tests, APS grades, and portal flow directions were evaluated before and after P-TACE sessions. Complications after procedures and survival days were also evaluated.
Results
In group A, APS grade was improved in eight patients and five of six patients with hepatofugal flow showed restored hepatopetal flow postoperatively. No immediate complication was developed in either group. Transient hepatic insufficiency developed in eight (42.1%) of 19 patients after P-TACE, and seven (87.5%) of these eight recovered within two weeks under conservative care. The mean and median survival time all study subjects was 280 days and 162 days.
It has been reported that arterioportal shunts (APS) of the liver can be caused by cirrhosis, metastases, hemangioma, hemangioendothelioma, hepatic trauma, Budd-Chiari disease, or hepatocellular carcinoma (HCC) (1). APS associated with HCC has been reported in about 28.8% to 63.2% of HCCs (1, 2), and is considered a poor prognostic factor for following reasons. First, the shunt flow to the portal vein aggravates the portal venous pressure, which induces life-threatening conditions such as esophageal varix, ascites, and hepatic encephalopathy (1, 3). Secondly, during transarterial oily chemoembolization (TACE), which has been used for many years to treat unresectable HCC (4), oil emulsion may be diverted into the portal vein branches and delivered to non-tumor hepatic tissue instead of being deposited intratumorally (1), and may induce severe adverse effects such as hepatic infarction. Therefore, we consider that APS should be managed before TACE.
A number of reports have been issued on APS embolization by coils, gelatin sponge, a combination of coils and gelatin sponge, ethanol, and polyvinyl alcohol (PVA) (3, 5-7). Coil embolization can result in the collateralization and restoration of flow into the vascular territory of the embolized vessels due to proximal embolization. Additionally, when proximal occlusion occurs, repeated interventions, which are common in HCC chemoembolization, via the same artery become difficult (8). Moreover, gelatin sponge is absorbed completely and the embolized vessels are recanalized within a few weeks of intervention (8, 9). Ethanol produces scarring of the bile duct, and results in sclerosing cholangitis in an animal experimental study (10). In contrast, PVA is not absorbable and it is more likely to produce a permanent occlusion because of the low frequency of recanalization. Moreover, as a particulate embolizing agent, it is easy to handle, and is available in a wide range of sizes (7-9). Although APS embolization with PVA and following chemoinfusion has been previously described (7), to the best of our knowledge, no systemic review is available on APS embolization followed by TACE.
We embolized APS using PVA before TACE (P-TACE) to prevent diversion of oily emboli from the hepatic artery to the portal vein and thus the resultant adverse effects. This retrospective study was conducted to evaluate the feasibility and safety of P-TACE for advanced HCC with APS.
Nineteen consecutive patients with unresectable HCC and APS who underwent P-TACE were selected from among 414 HCC patients between 1997 and 2004. The patients with a Child-Pugh class C hepatic function, or who underwent any treatments other than TACE throughout the study period were excluded from the evaluation. We obtained informed consents for about the procedures from all the patients. These 19 patients were classified into two groups. Group A (n = 10), patients who underwent 1 or 2 follow-up angiographies repeat TACE or P-TACE after the initial P-TACE. During the follow-up period, both TACE and P-TACE were performed in four patients, TACE in five, and P-TACE in one. Group B patients (n = 9) underwent only the initial P-TACE, because underlying liver cirrhosis and/or HCC had progressed too far to perform a repeated procedure or because no recurrence was demonstrated by follow-up computed tomography (CT).
The patient's ages ranged from 42 to 64 in group A (mean age: 51) and from 48 to 80 in group B (mean age: 62). Male to female ratio were 8:2 in group A and 7:2 in group B.
Initial hepatic functions were evaluated by using the Child-Pugh classification, and by determining serum bilirubin concentrations before and after P-TACE. Okuda stage, tumor size, APS grade, presence and extent of portal vein thrombosis, portal vein flow direction, and cavernous transformation were also evaluated on the preprocedural CT and angiographic images by two radiologists by consensus (Tables 1, 2). We semi-quantitatively classified APS from grade 0 to 3 (Table 3). 18 of the 19 patients (95%) had tumors larger than 10 cm in maximum diameter (Table 1). In group A, the initial APS were grade three in nine patients and grade 2 in one patient. In group B, the initial APS grade were grade 1 to 3 in three patients apiece.
Superior mesenteric arterioportography and hepatic arteriography were performed on all patients to evaluate portal veins and tumors. Angiograms obtained after an iopromide injection (Ultravist 370; Schering Korea, Seoul, Korea) were performed for superior mesenteric arterioportography at a flow rate of 4 ml/sec for 8 seconds and for hepatic arteriography at a rate of 4-5 ml/sec for 3 seconds. P-TACE was performed in two steps. The first step involved APS embolization with PVA (Contour; Boston Scientific, Natick, MA). PVA particle sizes used for the embolization were variable (150-1000 µm, Table 1). Although 355-500 and 500-710 µm PVA particles were used for APS embolization throughout the study period, smaller particles of 150-250 and 250-355 µm were used in two patients during the early period and larger particles (710-1000 µm), alone or mixed were used in the cases with extensive APS. When an APS had a single or two feeders, P-TACE was selectively performed at the level of the second or third order hepatic arteries with using a 4 Fr catheter or a microcatheter (n = 3, Table 1). However, the first order hepatic arteries of one or both hepatic lobes were embolized in most of cases (Table 1). When APS grade was converted to grade 0 by PVA embolization, conventional TACE with oil emulsion and gelatin sponge (Gelfoam; The Upjohn, Kalamazoo, MI) particles (size, 1 mm×1 mm×1 mm) was performed as a second step. When parasitic blood supply was detected, the same procedure was performed. Lobes or segments that contained tumors without APS were treated by TACE only (n = 2). Oil emulsions were prepared by mixing of 2-10 mg chemotherapeutic drug (Mitomycin C; Choong Wae Pharma Corp, Seoul, Korea) and 2-20 ml iodized oil (Lipiodol; Guerbet, Aulnay-sousBois, France) as previously described (4). In cases with hepatofugal portal flow by preprocedural arterioportography, portal flow was evaluated by superior mesenteric arterioportography after chemoembolization.
Follow-up examinations were conducted by CT with 1-3 months of initial intervention according to tumor extent and laboratory findings. Follow-up intervention was performed when a tumor recurred or remained viable by CT. When APS was severer than grade 0 was observed by preprocedural arteriography, P-TACE was repeated and when APS was grade 0, conventional TACE was performed without PVA embolization.
Feasibility was assessed using APS grades and the restoration of hepatopetal flow. APS grades were assessed on the pre- and postprocedural arteriograms of the hepatic and/or parasitic artery. Portal venous flow direction change was determined by superior mesenteric arterioportography before and immediately after P-TACE or TACE.
Procedural safeties were assessed by documenting the development of hepatic insufficiency or other complications, and by reviewing medical records, blood chemistry results, and image findings.
Hepatic insufficiency was defined when one or both of the following factors were positive after a procedure; a postprocedural Child-Pugh score higher than the preprocedural score (2 or more), or a serum bilirubin concentration greater than the preprocedural value (1 mg/dL or more). We defined irreversible hepatic insufficiency as a failure to recover blood chemistry values and clinical features within four weeks after P-TACE and when these factors were recovered within four weeks, we regarded the hepatic insufficiency as transient.
Arterioportal shunt grades were improved in eight (80%) patients in group A by last follow-up preprocedural arteriography (Fig. 1, Table 1). One patient (patient 10) with grade 3 APS failed to respond twice to P-TACE. Another patient (patient 9) showed a transient APS improvement from grade 3 to 0 on the preprocedural arteriogram at the second procedure, but APS was aggravated to grade 3 at third follow-up preprocedural arteriography (Fig. 2).
Six (60%) patients in group A showed hepatofugal flow on the initial preprocedural arteriograms. All of these patients were converted to hepatopetal flow immediately after P-TACE. On the second preprocedural arteriograms, one patient (patient 6) had recurred partial hepatofugal flow which was again converted to hepatopetal flow after the second P-TACE session. This patient maintained hepatopetal flow on the third follow-up preprocedural arteriogram. Another patient (patient 9) whose hepatofugal flow had been controlled after initial P-TACE had recurrent hepatofugal flow on the third follow-up procedural arteriogram. The hepatofugal flows that recurred in these two patients were produced by recurred APS after P-TACE (Fig. 3). Two patients (patient 4 and 15), one in each group, had no viable tumor on follow-up CT (Table 1).
None of the patients developed immediate complications. Postembolization syndrome manifested as fever (≥ 38℃, n = 17, 68%) abdominal pain (n = 4, 16%), and nausea (n = 4, 16%), but all of these postembolization syndromes were controlled by analgesics and antipyretics within a week.
Hepatic insufficiency was developed in nine (47%) of the 19 patients, eight (42.1%) after P-TACE and one (5.3%) after TACE (Table 1). All but one of the patients recovered within two weeks under conservative care. One patient (patient 14) developed irreversible hepatic insufficiency after P-TACE (Table 1). Serum bilirubin in this female patient had been 1 mg/dL before the initial P-TACE and subsequently increased to 5.7 mg/dL at two weeks and to 4.5 mg/dL at four weeks post P-TACE, but her hyperbilirubinemia maintained a plateau after four weeks. She died due to underlying HCC progression at 114 days after P-TACE.
Mean and median survivals were 400 and 256 days in group A, and 148 and 102 days in group B, respectively. Overall mean and median survivals were 280 and 162 days (Fig. 4). In group A, median survivals of patients that underwent P-TACE or TACE twice (n = 5) or three times (n = 5) were 206 and 274 days, respectively. Initial Okuda stages were higher in group B than group A (Table 1, p <0.05).
Recently, APS embolization has been attempted using different embolic materials (3, 5-7). Furuse et al. (5) performed APS embolization in 10 HCC patients without main portal vein thrombosis using coils in combination with gelatin sponges and achieved a median survival of 4.3 months. Tarazov et al. (6) also performed APS embolization using coils and gelatin sponges in five patients with HCC and APS. No serious complications were encountered in these two studies. The median survival observed in the present study (162 days) is similar with that of Furuse et al. (5). 95% of patients in our study had tumors larger than 10 cm, and 47% had main portal vein thrombosis. We attribute this relatively short survival to advanced tumors rather than to poor response to P-TACE. Moreover, the above studies did not provide details of tumor volumes and main portal vein thrombosis, which makes comparisons difficult.
Furuse et al. (5) used gelatin sponges and coils for APS embolization and performed follow-up Doppler ultrasonography, 1-2 weeks after embolization. They reported APS regression and portal flow restoration after APS embolization. However, we believe that this follow-up period is too short to evaluate the effect of APS embolization because gelatin sponge absorption, which usually occurs 35-45 days after embolization (9), can cause APS recanalization and thus portal flow reversal. Therefore, we consider that the results by Furuse et al. may be overestimated.
Huang et al. (3) performed ethanol embolization in HCC patients and severe APS, and compared this with gelatin sponge embolization. They reported a median survival of 11 months, which is much longer than the 162 days of our study. We suppose that this survival difference reflects tumor stage and shunt severity differences. However, no mention was made of portal flow which would have provided an indication of shunt severity. Moreover, because small amounts of ethanol (2-6 ml) were used after selecting APS feeding vessels, we believe that the tumors were not those of advanced HCC. Because most of our cases had large tumors, P-TACE had to be performed at the hepatic artery proper or at first order branches. Moreover, ethanol embolization is associated with severe adverse effects, such as sclerosing cholangitis induced by bile duct scarring as was reported by an experimental study (10), although they did not encounter this complication. Another disadvantage of ethanol is that it is more difficult to handle than particulate PVA.
There are some intrinsic reasons that favor the use of PVA for APS embolization. PVA is not absorbable and thus is likely to produce long-term occlusion because recanalization is more difficult than for gelatin sponge and/or coils (7-9). PVA particles are small as compared with coils and gelatin sponge particles, so distal embolization, which is mandatory to tumor embolization, is possible (11). In addition, PVA particles are easier to handle.
In a previous study on APS embolization using PVA in patients with HCC and portal vein thrombosis (7), 55% of the patients showed APS improvements after first embolization when PVA was used, which is similar to our result (60%) after the initial P-TACE. Moreover we were able to control APS in 80% by repeated P-TACE, when APS remained or recurred in patients with a low risk of hepatic failure. These results show that repeated APS embolization using PVA is highly effective, although it should be mentioned that two patients (20%) did not respond to the P-TACE.
Two patients showed no viable tumor by follow-up CT. One of these patients remains alive for 809 days after P-TACE without evidence of tumor recurrence (Fig. 1), and the other lived for 500 days. Although the factors that predict response to P-TACE have not been identified, it is evident that P-TACE can be an efficacious treatment in some patients with APS accompanying HCC. However, P-TACE may induce complete occlusion of tumor feeding arteries, which reduces the amount of oil emulsion and hence cytotoxic drug to administrated during chemoembolization. We administrated oil emulsion when an APS of grade 0 had been attained by PVA embolization. On the follow-up CT, tumors were found to retain lipiodol, but the amount of retention was not as great as in HCC without APS, which means that tumor feeding vessels were not completely occluded by PVA embolization. Therefore, we hypothesized that APS may have been occluded earlier than tumor feeding arteries due to high flow of the arteries to APS.
The control of hepatofugal portal flow is vital to reduce severe manifestations of portal hypertension and to prevent migration of administered medication and oily emboli via collaterals from the portal to the systemic veins during TACE. Thus, we embolized APS with PVA before routine TACE to prevent medications and oily emboli from migrating into the non-targeted hepatic tissue and systemic veins (12). In this study, although hepatofugal flow was not amenable to P-TACE in two cases, we view PVA embolization as a highly effective means of controlling hepatofugal flow associated with an APS.
In contrast to our study which involved APS embolization and TACE, Lee et al. (7) performed APS with chemoinfusion probably due to the concern of hepatic insufficiency. They reported two cases of hepatic failure, though a definition and the timings of these failures were not mentioned. In our study, eight (42.1%) of 19 patients manifested hepatic insufficiency by P-TACE, but only one patient developed irreversible hepatic insufficiency despite a relatively good preprocedural hepatic function. However, although this patient fell into the category of irreversible hepatic insufficiency, her hepatic failure was not progressive. Therefore, we view that the incidence of the post P-TACE hepatic failure (5.3%, 1/19 patients) was low. We excluded patients with an accompanying poor hepatic function or complete occlusion of the main portal vein without cavernous transformation, because these patients are apt to develop hepatic insufficiency and we presume that this is an explanation of the low incidence of irreversible hepatic insufficiency. In the present study, the frequency of postembolization syndrome occurrence was similar to those of other studies (5, 7). However, it was not clear whether postembolization syndrome was induced by PVA embolization or TACE.
Okuda stages of the patients were poorer in group B than that of in group A. We believe that this led to low survival in group B excepting the patient who survived for 500 days without additional P-TACE or TACE. Another possible reason for the observed poor survival of group B might have been a poor response to P-TACE. However, we believe that the initial clinical status played a major role in survival.
This study has some limitations. The number of the patients enrolled was small. Follow-up periods were relatively short as most of patients had advanced disease. Retrospective nature prevented comparisons with other forms of embolization or a control group. In terms of the treatment of HCC, chemoembolization is usually not considered for advanced tumors with APS, because nontarget embolization via APS may induce severe complications like infarction. However, the results of the present study suggest that P-TACE can control APS safely and effectively and demonstrate enhanced control of APS associated with HCC.
We conclude that P-TACE appears to be a feasible and safe means of treating APS associated with advanced HCC.
Figures and Tables
Fig. 1
A 43-year-old male with grade 3 arterioportal shunts and hepatocellular carcinomas (patient 4).
A-F. Initial CT (A) revealed a large poorly enhanced mass in the left lobe of a cirrhotic liver and left portal vein thrombosis. Superior mesenteric arterioportogram (B) showing extensive collaterals via the coronary vein and an absent main portal vein. Hepatic arteriogram (C), showing tumor staining and extensive arterioportal shunts (black arrows) in the left hepatic lobe. The main portal vein was opacified by hepatofugal flow. P-TACE (355-500 µm, 1 bottle) was administered to the left hepatic and middle hepatic arteries. Left and middle hepatic arteries were occluded by P-TACE (D). Follow-up superior mesenteric arterioportogram (E) obtained two months after P-TACE, showing restored hepatopetal flow. Hepatic arteriogram (F), showing a non-opacified arterioportal shunts. G, H. Follow-up CT scan (G), six months after P-TACE, showing some lipiodol retention without viable tumor. The tumor and lipiodol retention were not observed by CT (H), 22 months after P-TACE.
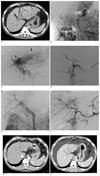
Fig. 2
A 51-year-old woman with grade 3 arterioportal shunt and hepatocellular carcinomas (patient 9). On initial hepatic arteriogram (A) showing multiple tumors in both hepatic lobes (black arrows). The main portal vein (white arrow) was opacified indirectly through arterioportal shunt of hepatocellular carcinomas. Polyvinyl alcohol embolization was performed using half a bottle of 355-500 µm sized particles via the right hepatic artery and conventional TACE was performed in both hepatic lobes. Follow-up hepatic arteriogram (B), two months after P-TACE, demonstrating arterioportal shunt improvement. Transarterial chemoembolization was performed without polyvinyl alcohol embolization. After four months, arterioportal shunt recurred in both hepatic lobes with hepatofugal flow in the main portal vein (arrow) on gastroduodenal (C) and right inferior phrenic (D) arteriograms. P-TACE was performed using one bottle of 355-500 µm sized particles into both arteries.
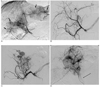
Table 1
Clinical Data and Outcomes of P-TACE

Note.-PVA = polyvinyl alcohol, Before = before procedure, After = after procedure, 2nd = second order branch of the hepatic artery, 3rd = third order branch of the hepatic artery, RHA = right hepatic artery, LHA = left hepatic artery, BHA = both hepatic arteries, GDA = gastroduodenal artery, RIPA = right inferior phrenic artery.
A = 150-250 µm, B = 250-355 µm, C = 355-500 µm, D = 500-710 µm, E = 710-1000 µm,.
*Vessels of polyvinyl alcohol embolization, **Oily chemoembolization without polyvinyl alcohol embolization, †Living patient, ‡Irreversible hepatic insufficiency
References
1. Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997. 52:36–40.
2. Okuda K, Musha H, Yamasaki T, Jinnouchi S, Nagasaki Y, Kubo Y, et al. Angiographic demonstration of intrahepatic arterioportal anastomoses in hepatocellular carcinoma. Radiology. 1977. 122:53–58.
3. Huang MS, Lin Q, Jiang ZB, Zhu KS, Guan SH, Li ZR, et al. Comparison of long-term effects between intra-arterially delivered ethanol and Gelfoam for the treatment of severe arterioportal shunt in patients with hepatocellular carcinoma. World J Gastroenterol. 2004. 10:825–829.
4. Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983. 148:397–401.
5. Furuse J, Iwasaki M, Yoshino M, Konishi M, Kawano N, Kinoshita T, et al. Hepatocellular carcinoma with portal vein tumor thrombus: embolization of arterioportal shunts. Radiology. 1997. 204:787–790.
6. Tarazov PG. Intrahepatic arterioportal fistulae: role of transcatheter embolization. Cardiovasc Intervent Radiol. 1993. 16:368–373.
7. Lee DH, Yoon HK, Song HY, Kim GC, Hwang JC, Sung KB. Embolization of severe arterioportal shunts in the patients with hepatocellular carcinoma: safety and influence on patient survival. J Korean Radiol Soc. 1999. 41:1117–1125.
8. Coldwell DM, Stokes KR, Yakes WF. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994. 14:623–643.
9. Siskin GP, Englander M, Stainken BF, Ahn J, Dowling K, Dolen EG. Embolic agents used for uterine fibroid embolization. AJR Am J Roentgenol. 2000. 175:767–773.
10. Doppman JL, Girton ME. Bile duct scarring following ethanol embolization of the hepatic artery: an experimental study in monkeys. Radiology. 1984. 152:621–626.
11. Bendszus M, Klein R, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. AJNR Am J Neuroradiol. 2000. 21:255–261.
12. Wachsberg RH, Bahramipour P, Sofocleous CT, Barone A. Hepatofugal flow in the portal venous system: pathophysiology, imaging findings, and diagnostic pitfalls. Radiographics. 2002. 22:123–140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download