Abstract
Objective
The aim of this study was to examine the incidence of ischemia during protected carotid artery stenting (CAS) as well as to compare the protective efficacy of the balloon and filter devices on diffusion-weighted MR imaging (DWI).
Materials and Methods
Seventy-one consecutive protected CAS procedures in 70 patients with a severe (> 70%) or symptomatic moderate (> 50%) carotid artery stenosis were examined. A balloon device (PercuSurge GuardWire) and a filter device (FilterWire EX/EZ, Emboshield) was used in 33 cases (CAS-B group) and 38 cases (CAS-F group) to prevent distal embolization, respectively. All the patients underwent DWI within seven days before and after the procedures. The number of new cerebral ischemic lesions on the post-procedural DWI were counted and divided into ipsilateral and contralateral lesions according to the relationship with the stenting side.
Results
New cerebral ischemic lesions were detected in 13 (39.4%) out of the 33 CAS-Bs and in 15 (39.5%) out of the 38 CAS-Fs. The mean number of total, ipsilateral and contralateral new cerebral ischemic lesion was 2.39, 1.67 and 0.73 in the CAS-B group and 2.11, 1.32 and 0.79 in the CAS-F group, respectively. No statistical differences were found between the two groups (p = 0.96, 0.74 and 0.65, respectively). The embolic complications encountered included two retinal infarctions and one hemiparesis in the CAS-B group (9.09%), and one retinal infarction, one hemiparesis and one ataxia in the CAS-F group (7.89%). There was a similar incidence of embolic complications in the two groups (p = 1.00).
Carotid artery stenting (CAS) is a comparative method for treating a carotid artery stenosis. However, the occurrence of distal embolization with the procedure is still a major concern because friable plaque can be separated from the diseased vessel wall during the procedure. Therefore, several protection devices have been developed and applied to the prevention of a distal embolization. The use of filter devices by interventional neuroradiologists has increased recently to the point that their use has exceeded that of balloon devices due to technical convenience of the former. However, safety of the two different protection devices has not been fully tested. Therefore, the use of the filter device is not justified.
Diffusion-weighted MR imaging (DWI) is very sensitive and specific technique for diagnosing cerebral ischemia (1-3). DWI is widely accepted as a marker of ischemic complications in many interventional and surgical procedures (1, 4-11). Hammer et al. examined the incidence of new DWI lesions after protected CAS with two types of distal filter devices (12). In that study, new focal ischemic lesions were detected on DWI in 40% of the procedures. Ninety percent of these events were clinically silent and 62% occurred outside the vascular territory of the treated side. Du Mesnil de Rochemont et al. also detected new DWI lesions in 14 (28%) out of 50 cases in the territory of the stented internal carotid artery and in seven (14%) out of 50 cases in other vascular territories after protected CAS with a distal filter device (13). Asakura et al. used DWI to compare the two types of embolic protection methods during CAS, and reported better protection with a simultaneous double occlusion of both the internal and external carotid arteries than with the single protection of the internal carotid artery (ischemic spots on DWI; 36.0% vs. 55.0%, respectively) (14). Asakura et al. also demonstrated excellent protection using a flow reversal device (Parodi Anti-Emboli System) during CAS by DWI (ischemic spots on DWI; 18.2%) (15). However, there has been no comparison of the number of new DWI lesions formed after protected CAS using the distal balloon with that using the filter device.
In this study, the occurrence of cerebral embolization after protected CAS in a balloon device group (CAS-B) and a filter device group (CAS-F) was examined and compared using DWI.
From May 2002 to October 2005, 73 protected CASs were performed in 72 patients with a severe (> 70%) or symptomatic moderate (> 50%) carotid artery stenosis using a distal balloon or a filter device prior to the deployment of the stent. Among these patients, two patients, who had undergone CAS-B, were excluded due to intolerance that developed while inflating the balloon device. The remaining 70 patients consisted of 59 males and 11 females with a mean age of 66.5 years (age range: 43 to 84 years). Among the 71 CAS procedures, 35 and 36 CAS procedures were performed in the right and left carotid artery, respectively. Bilateral CAS was performed in one patient with a 15-month interval. Four types of selfexpandable stents were used. The SMART (Cordis Corp, Miami, FL), PRECISETM RX (Cordis Corp., Miami, FL), Carotid WALLSTENTTM MonorailTM (Boston Scientific Corp., Galway, Ireland), and Zilver 518 (William COOK Europe ApS, Bjaeverskov, Denmark) stents were used in 28, 25, 17 and one procedures, respectively. During the early period from May 2002 to February 2004, one type of balloon device, the PercuSerge GuardWire system (Medtronic, Danvers, MA) was used in 33 procedures (CAS-B), and three kinds of filter device, the FilterWire EXTM (Boston Scientific Corp, Natick, MA), FilterWire EZTM (Boston scientific Corp., Natick, MA), and Emboshield filter protection systems (MedNova, Galway, Ireland), were used in seven, 23 and eight procedures during late period from March 2004 to October 2005, respectively (CAS-F).
One of three experienced interventional neuroradiologists performed the procedures. DWI was obtained from all patients within one week before the procedures and between 24 hours to one week after the procedures. Two neuroradiologists counted and recorded the number of new high signal intensities (HI) in the cerebral hemispheres by comparing the pre- and post-procedural DWIs for each CAS procedure without any knowledge of the clinical status of the patients or the types of protection devices used. The new HIs were divided into the ipsilateral or contralateral type according to the relationship with the treated side. The following information for each patient was also collected: age, gender, degree of ipsilateral and contralateral carotid artery stenosis, presenting symptoms, HbA1c value in the patients with diabetes mellitus, ulceration (presence of contrast or hazy contour beyond the vessel lumen) of the carotid artery stenosis on the baseline angiogram and any change in the neurological status at the time of DWI acquisition. The degree of carotid artery stenosis was calculated based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria.
Although not established, some risk factors have been suggested as a predictor of complications after CAS (13, 16-30). Therefore, the following variables, which were used to create a risk score for peri-interventional complications after CAS, were defined as risk factors in this study (30): diabetes mellitus with inadequate glycemic control (HbA1c > 7%), age ≥ 80 years, ulceration of the carotid artery stenosis and a contralateral stenosis ≥ 50%. Written informed consent was obtained from all the patients before the procedure.
The difference in the number of new cerebral ischemic lesions between the two groups was evaluated using a Mann-Whitney test. The incidence of clinically apparent neurological complications in the two groups was compared using a Pearson's χ2 test. In addition, variables such as age and the degree of the ipsilateral and contralateral carotid artery stenosis in the two groups were compared using an independent t-test or Mann-Whitney test, and the presenting symptoms were compared using a Pearson's χ2 test. The difference in the number of new cerebral ischemic lesions according to the three type of filter devices used was compared using a Kruskal Wallis test. For all the tests in this study, p values < 0.05 were considered significant. All the statistical analyses were performed using the SPSS software program (SPSS 10.0; SPSS, Chicago, IL).
All the patients were administered clopidogrel (75 mg/day) and aspirin (100 mg/day) at least 72 hours before the CAS procedure. An 8 or 9 Fr sheath (Cook Inc., Bloomington, IN) was inserted into the common femoral artery for vascular access, and a 6 Fr sheath (Cook Inc., Bloomington, IN), as temporary cardiac pacemaker, was inserted into the common femoral vein. Intravenous heparin (80 IU/Kg) was injected as a bolus and then dripped continuously during the procedure to maintain a two or three times elongated activated clotting time. A 5 Fr neuroangiographic catheter was introduced into the target lesion-related common carotid artery, and a 300-cm 0.035/0.038-inch exchange guidewire (Terumo Corp., Tokyo, Japan) was advanced into the external carotid artery. The 5 Fr neuroangiographic catheter was then replaced with either a 7 Fr carotid sheath (Shuttle Flexor; Cook Inc., Bloomington, IN) or an 8 Fr guiding catheter (VistaBrite tip; Cordis Corp., Miami, FL). The appropriate length and diameter stent was determined according to the degree and length of the stenotic segment and the diameter of carotid artery immediately below the bifurcation measured on the baseline angiogram. After passing through the stenotic segment, a balloon or filter type distal protection device was deployed in the distal cervical or proximal petrosal internal carotid artery. The stenotic lesions were pre-dilated with a 3- or 4-mm diameter balloon catheter (Symmetry/Ultra-soft SV; Boston Scientific Corp., Natick, MA, or Amiia/Savvy; Cordis Europa N.V., Roden, Netherlands), with the exception of 14 cases. After this or as a primary dilatation procedure, a self-expandable carotid stent was placed across the stenosis. The residual stenotic segment was then postdilated using a 5- or 6-mm diameter balloon catheter (Symmetry, Amiia, or Savvy) with the exception of two cases. In the case of using a balloon device, the protective balloon occlusion, the procedures for aspirating the debris and balloon deflation were repeated in each step. The balloon dilation was not performed more than twice, or for longer than 10 seconds in duration. A completion angiogram was obtained after removing the protection device.
DWI was obtained using a 1.5 T MR imaging apparatus (Signa Horizon or Signa CV/I; GE Medical Systems, Milwaukee, WI) or a 3.0 T MR imaging apparatus (Intera Achieva 3.0T; Philips Medical Systems, Best, Netherlands). Besides DWI, the other sequences were obtained, which included the axial spin echo T1 weighted image, the fast spin echo T1 image, the fast spin echo T2 weighted image, the FLAIR (fluid-attenuated inversion-recovery) image, the perfusion image and the contrast enhanced spin echo T1 weighted image. DWI was acquired with the echo-planar method. The TR/TE/NEX, field of view, the matrix and bvalues were 6500/97/1, 280 mm, 128×128, 0 and 1000 s/mm2, respectively, for the 1.5 T MR apparatus, and 3421.3/60/1, 24 mm, 176×176, 0 and 1000 s/mm2 for the 3.0 T MR apparatus, respectively.
All the CAS procedures were technically successful. Table 1 gives a list of the baseline characteristics of the patients. Among several variables, only the degree of contralateral carotid artery stenosis showed a significant difference between the CAS-B and CAS-F groups (the mean value was 37.2 vs. 19.0, respectively, p = 0.01). In addition, there was a significant difference in the incidence of severe stenosis (> 70%) or occlusion of the contralateral carotid artery between the two groups (6 vs. 1; p = 0.04, Pearson's χ2 test). The mean time from the procedure to the acquisition of the pre- and post-procedural DWI in the CAS-B group was 2.3 and 1.2 days, respectively (range: 0-7 and 1-3 days, respectively), and 2.7 and 1.4 days, respectively (range: 0-7 and 1-4, respectively) in the CAS-F group. The differences between the two groups were not significant (p = 0.59 and 0.12, respectively, Mann-Whitney test).
A new HI on the post-procedural DWI was detected in 39.4% and 39.5% of the CAS-B patients and CAS-F patients, respectively (Figs. 1, 2). The diameter of all new high SI lesions on DWI was several millimeters and they did not exceed 1 cm. There were no apparent major arterial or territorial infarctions observed. The total numbers of new ipsilateral and contralateral HIs were 55 and 24 in the CAS-B group, and 50 and 30 in the CAS-F group. The mean numbers of ipsilateral, contralateral and total new HIs were 1.67 (range: 0-31), 0.73 (range: 0-7), and 2.39 (range: 0-32), respectively, in the CAS-B group, and 1.32 (range: 0-16), 0.79 (range: 0-9), and 2.11 (range: 0-16), respectively, in the CAS-F group. Between the two groups, there was a similar number of ipsilateral, contralateral and total new HIs (p = 0.74, 0.65 and 0.96, respectively, Mann-Whitney test) (Table 2). When the new HIs were divided into cortical/juxta-cortical white matter, deep periventricular white matter and deep gray matter according to their location, there were 64, 11 and four new HIs in the CAS-B group, respectively, and 67, nine and four in the CAS-F group, respectively. Although the process and protective mechanism of the two devices were different, there was no significant difference in the distribution of the new HIs between the two groups and almost all infarctions appeared to result from a thromboembolism.
The mean numbers of ipsilateral, contralateral and total new HIs for each of the three types of filter devices were as follows: 0.43, 0.14 and 0.57 in the Filter Wire-EX system, respectively, 0.87, 1.00 and 1.87 in the Filter Wire-EZ system, respectively, and 3.38, 0.75 and 4.13 in the Emboshield filter protection system, respectively. There was no significant difference between the three groups (p = 0.95, 0.83 and 0.87, respectively, Kruskal-Wallis test) (Table 3).
There were two cases of retinal artery embolisms and one case of hemiparesis occurred in the CAS-B group, and one case of retinal artery embolism, one case of hemiparesis and one case of ataxia in the CAS-F group. In addition, one case of hyperperfusion syndrome occurred in the CAS-F group. The symptoms such as hemiparesis and ataxia corresponded to the distribution of the new HIs on DWI. The retinal embolism was demonstrated after the procedure as a typical symptom and/or a loss of choroidal blush immediately. For two of the three patients with retinal embolisms, direct or indirect retinal artery thrombolysis was performed and one retinal embolism was successfully recanalized. Among the three patients with a retinal embolism, two patients developed visual field defects and one patient developed blindness. The overall embolic complications such as retinal embolism, hemiparesis and ataxia were less frequent in the CAS-F group (7.89%) than in the CAS-B group (9.09%). However, there was no significant difference between the two groups (p = 0.61, Fisher's exact test).
The mean number of risk factors was 1.30 in the CAS-B group (range: 0-3) and 0.76 in the CAS-F (range: 0-2). The number of risk factors was significantly higher in the CAS-B group than in the CAS-F (p = 0.02, Mann-Whitney test). Twenty-five CAS-Bs and 23 CAS-Fs were categorized into a high-risk group with at least one risk factor, and eight CAS-Bs and 15 CAS-Fs were categorized into a low-risk group without any of the risk factors. In both the high and low risk groups, there was a similar number of ipsilateral, contralateral and total new HIs in the CAS-B and the CAS-F groups (Table 4). In the high-risk group, there were two cases of retinal artery embolisms and one case of hemiparesis in the CAS-B, and one case of hemiparesis in the CAS-F. In the low risk group, there were no embolic complications in the CAS-B, and one case of retinal artery embolism and one case of hemiparesis in the CAS-F. In both the high and low risk groups, there was a similar incidence of embolic complications in the CAS-B and the CAS-F (p = 0.61 and 0.53, respectively, Fisher's exact test).
Carotid artery stenting has now been highlighted as an alternative treatment to carotid endarterectomy (CEA) because it is less invasive and it has a larger capability for high-risk patients than CEA. According to the CAVATAS (initial randomized multicenter clinical trial of Carotid and Vertebral Transluminal Angioplasty Study), an endovascular treatment using stents or balloons showed similar effectiveness in the treatment of carotid artery stenosis to CEA (31). Although a previous study at our institution showed a significantly higher rate of ischemic brain lesions on DWI after unprotected CAS than after CEA, CAS appeared to be a comparable method to conventional CEA when considering the overall symptomatic complications (32). The SAPPHIRE trial (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy trial), which was the first controlled randomized trial of CAS using a protection device, suggested that CAS using an embolic protection device was not inferior to CEA and has a lower incidence of 30 day adverse clinical events compared with CEA (33). Ouriel et al. reported a multicenter feasibility trial of carotid artery stenting with and without an embolus protection catheter, and concluded that the use of an embolus protection device might reduce the risk of postprocedural major ipsilateral strokes (34).
The concept of cerebral protection during CAS was first introduced through the modification of a protection device that was originally used in saphenous vein graft intervention, and the effectiveness of a cerebral protection device was first mentioned in 1990 (35, 36). Three methods are currently used for cerebral protection. These include distal balloon devices, distal filter devices and proximal occlusion catheters. Each device has its own advantages and disadvantages.
Any distal protection device, either a filter or balloon, needs to pass through the stenotic portion before being positioned in the distal internal carotid artery, and the catheter passage itself might provoke distal embolization. Minor movement of the inflated balloon or deployed filter may also cause internal injury. Balloon devices are easier to adapt to stenotic or tortuous vessels than filter devices. However, balloon dilatation might cause a dissection or spasm of the distal internal carotid artery. In addition, patients with the incomplete development of the circle of Willis may not tolerate any disruption in the internal carotid artery flow (37). In this series, such intolerance was encountered in two patients when the internal carotid artery (ICA) flow was disrupted after balloon occlusion. These patients were excluded from the study. After balloon dilatation, the disrupted blood flow proceeds into the external carotid artery, which may cause a retinal or cerebral embolization through the external-internal carotid or the external carotid-ophthalmic or the external carotid-vertebral potential anastomotic channels (38, 39). In contrast, filter devices do not completely disrupt the blood flow. However, they cannot prevent the transport of embolic particles smaller than the filter pore. Moreover, the process of filter retrieval itself might cause distal embolization. The proximal occlusion catheter does not need to cross the stenotic lesion and can capture all particle sizes. Unlike a distal balloon occlusion or filter device, the proximal occlusion catheter can induce reversed flow from the ICA and the external carotid artery (ECA) to the common carotid artery (CCA) by occluding the CCA. However, the proximal occlusion catheter can stop the antegrade cerebral blood flow possibly resulting in intolerance (40).
Among the three kinds of protection devices, the balloon devices and the filter devices are in general use. The use of a balloon device is now decreasing on account of its technical complexity. In contrast, the use of a filter device has increased to the point that it now exceeds that of the balloon device because the filter device is technically easier for interventional neuroradiologists to handle than balloon devices. However, the safety of the two different protection devices has not yet been fully tested.
In this study, the incidence of new DWI lesions after protected CAS was similar in the both groups (39.4% in the CAS-B group and 39.5% in the CAS-F group). Among these new DWI lesions, 30.4 % in the CAS-B group and 37.5 % in the CAS-F group were attributed to lesions contralateral to the treated side. Embolic complications such as retinal artery embolism, hemiparesis and ataxia, occurred only in 7.89% and 9.09% of the CAS-F and CAS-B group, respectively. Such a high incidence of socalled silent ischemic lesions and a large proportion of new ischemic lesions in the territory contralateral to the treated side corresponded to the results of a previous study that used two types of distal filter devices (12). Similar to their assumption, besides crossing and angioplasty in the targeted ICA, the handling at the level of the aortic arch or the common carotid artery before placing the protection device and diagnostic angiography at the contralateral ICA appeared to be the cause of the high incidence of new DWI lesions. Collateral flow through the anterior communicating artery also might be one of causative factors for a contralateral ischemic lesion.
Although the numbers of risk factors were significantly higher in the CAS-B group, the number of ipsilateral, contralateral and total new HIs were similar in both the high and low risk groups. In addition, the incidence of embolic complications such as a retinal artery embolism, hemiparesis and ataxia were similar in both risk groups in the CAS-B and the CAS-F groups.
There have been several comparative studies on the efficacy of the various protection devices. Zahn et al. compared the effectiveness of the balloon device in 176 patients and the filter device in 553 patients, and concluded that the occurrence of in-hospital death or stroke was similar in the two groups (2.3% vs. 1.8%, respectively) (41). Muller-Hulsbeck et al. compared the efficacy of four types of cerebral protection devices using an in vitro model and concluded that the NeuroShield filter and the FileterWire EX captured the highest percentage of human emboli (42).
There also have been several case series or trials on protected CAS using either a filter or balloon device. Table 5 summarizes the characteristics and 30-day outcomes of these studies (33, 34, 43-49).
This study had some limitations. First, the proportion of the four stent types was not identical in the CAS-B and CAS-F groups. Therefore, besides the different types of protection devices, the influence of the different stent types on the occurrence of new HI could not be completely excluded. Second, during the course of study, the balloon catheters available tended to decrease in diameter and improve in quality. Therefore, such improvements might also have influenced the results. Third, there was a significant difference in degree of contralateral carotid artery stenosis between the CAS-B and CAS-F groups (the mean value was 37.2 vs. 19.0, respectively, p = 0.01). Therefore, the effect of such a difference on the results also cannot be ignored.
In conclusion, the overall occurrence of new embolic cerebral lesions detected on DWI was higher than expected. The types of distal protection devices, i.e. balloon or filter, did not significantly affect the occurrence of cerebral embolization. The operator's decision after considering the individual strengths and weaknesses of the various protection devices that are appropriate to different circumstances appears to be important.
Figures and Tables
Fig. 1
A 72-year-old man who underwent protected carotid artery stenting with a balloon device.
A. Pre-stenting angiogram shows severe stenosis (86.3%) at the left internal carotid artery.
B. A balloon device is deployed in the distal carotid artery (arrow).
C. After carotid artery stenting, the lumen of the left internal carotid artery is successfully dilated.
D. No ischemic lesion is shown in bilateral cerebral hemispheres on the pre-stenting diffusion weighted MR imaging.
E. Multiple small new hyperintensities are shown on the post-stenting diffusion-weighted MR imaging. Note the new hyperintnesities are distributed in not only the ipsilateral but also the contralateral cerebral hemisphere. However, no symptomatic neurological complications occurred after carotid artery stenting.
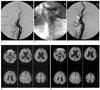
Fig. 2
A 71-year-old man who underwent protected carotid artery stenting with a filter device.
A. Pre-stenting angiogram shows a severe string like stenosis of the left internal carotid artery. The post-stenotic distal internal carotid artery is also narrow compared with the external carotid artery (pseudo-occlusion).
B. A filter device is deployed in the distal carotid artery (arrow).
C. After carotid artery stenting, the lumen of the left internal carotid artery is successfully dilated.
D. No ischemic lesion is shown in bilateral cerebral hemispheres on the pre-stenting diffusion weighted MR imaging.
E. Multiple small new hyperintensities are observed in the ipsilateral cerebral hemisphere on the post-stenting diffusion-weighted MR images. However, no symptomatic neurological complications occurred after carotid artery stenting.
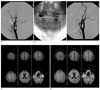
Table 3
Comparison of the New High Signal Intensities according to the Three Types of Distal Filter Devices

Table 4
Comparison of the New High Signal Intensities between the CAS-B and CAS-F Groups according to the Risk Categories

Table 5
Several Case Series or Trials for Protected Carotid Artery Stenting: Characteristics and 30-day Outcomes

Note.-MI = myocardial infarction
1NeuroShield (Mednova Inc., Galway, Ireland)
2GuardWire (Percusurge Inc., Sunnyville, CA)
3Angioguard (Cordis Inc., Miami, FL)
4FilterWire EX (Boston Scientific Corp. Natick, MA)
5Trap Filter (Microvena/EV3, Plymouth, MN)
6Percuserge (Medtronic Inc., Danvers, MA)
7Medicorp occlusive balloon (Medicorp Inc., Villers-les-Nancy, France)
8Angioguard (J & J Cordis Europe, Roden, The Netherlands)
9EPI Filter Wire EX (Boston Scientific Corp., Santa Clara, CA)
10FilterWire EZ (Boston Scientific Corp., Natick, MA)
11Interceptor Carotid Filter System (Medtronic Inc., Minneapolis, MN)
References
1. Lövblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol. 1998. 19:1061–1066.
2. Beauchamp NJ Jr, Barker PB, Wang PY, vanZijl PC. Imaging of acute cerebral ischemia. Radiology. 1999. 212:307–324.
3. van Everdingen KJ, van der Grond J, Kappelle LJ, Ramos LM, Mali WP. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998. 29:1783–1790.
4. Forbes KP, Shill HA, Britt PM, Zabramski JM, Spetzler RF, Heiserman JE. Assessment of silent embolism from carotid endarterectomy by use of diffusion-weighted imaging: work in progress. AJNR Am J Neuroradiol. 2001. 22:650–653.
5. Barth A, Remonda L, Lövblad KO, Schroth G, Seiler RW. Silent cerebral ischemia detected by diffusion-weighted MRI after carotid endarterectomy. Stroke. 2000. 31:1824–1828.
6. Feiwell RJ, Besmertis L, Sarkar R, Saloner DA, Rapp JH. Detection of clinically silent infarcts after carotid endarterectomy by use of diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001. 22:646–649.
7. Rordorf G, Bellon RJ, Budzik RE Jr, Farkas J, Reinking GF, Pergolizzi RS, et al. Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001. 22:5–10.
8. Muller M, Reiche W, Langenscheidt P, Hassfeld J, Hagen T. Ischemia after carotid endarterectomy: comparison between transcranial Doppler sonography and diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2000. 21:47–54.
9. Jaeger HJ, Mathias KD, Drescher R, Hauth E, Bockisch G, Demirel E, et al. Diffusion-weighted MR imaging after angioplasty or angioplasty plus stenting of arteries supplying the brain. AJNR Am J Neuroradiol. 2001. 22:1251–1259.
10. Britt PM, Heiserman JE, Snider RM, Shill HA, Bird CR, Wallace RC. Incidence of postangiographic abnormalities revealed by diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2000. 21:55–59.
11. Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999. 354:1594–1597.
12. Hammer FD, Lacroix V, Duprez T, Grandin C, Verhelst R, Peeters A, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg. 2005. 42:847–853. discussion 853.
13. du Mesnil de Rochemont R, Schneider S, Yan B, Lehr A, Sitzer M, Berkefeld J. Diffusion-weighted MR imaging lesions after filter-protected stenting of high-grade symptomatic carotid artery stenoses. AJNR Am J Neuroradiol. 2006. 27:1321–1325.
14. Asakura F, Kawaguchi K, Sakaida H, Toma N, Matsushima S, Kuraishi K, et al. Diffusion-weighted magnetic resonance imaging in carotid angioplasty and stenting with balloon embolic protection devices. Neuroradiology. 2006. 48:100–112.
15. Asakura F, Kawaguchi K, Sakaida H, Toma N, Matsushima S, Kuraishi K, et al. Diffusion-weighted MR imaging in carotid angioplasty and stenting with protection by the reversed carotid arterial flow. AJNR Am J Neuroradiol. 2006. 27:753–758.
16. Gröschel K, Ernemann U, Riecker A, Schmidt F, Terborg C, Kastrup A. Incidence and risk factors for medical complications after carotid artery stenting. J Vasc Surg. 2005. 42:1101–1106. discussion 1106-1107.
17. Henry M, Gopalakrishnan L, Rajagopal S, Rath PC, Henry I, Hugel M. Bilateral carotid angioplasty and stenting. Catheter Cardiovasc Interv. 2005. 64:275–282.
18. Hobson RW, Howard VJ, Roubin GS, Brott TG, Ferguson RD, Popma JJ, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004. 40:1106–1111.
19. Kastrup A, Gröschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003. 34:813–819.
20. Kastrup A, Gröschel K, Schulz JB, Nägele T, Ernemann U. Clinical predictors of transient ischemic attack, stroke, or death within 30 days of carotid angioplasty and stenting. Stroke. 2005. 36:787–791.
21. Krapf H, Nägele T, Kastrup A, Bühring U, Grönewäller E, Skalej M, et al. Risk factors for periprocedural complications in carotid artery stenting without filter protection: a serial diffusion-weighted MRI study. J Neurol. 2006. 253:364–371.
22. Lanzer P, Weser R, Prettin C. Carotid-artery stenting in a high-risk patient population-single centre, single operator results. Clin Res Cardiol. 2006. 95:4–12.
23. Reiter M, Bucek RA, Effenberger I, Boltuch J, Lang W, Ahmadi R, et al. Plaque echolucency is not associated with the risk of stroke in carotid stenting. Stroke. 2006. 37:2378–2380.
24. Roubin GS, New G, Iyer SS, Vitek JJ, Al-Mubarak N, Liu MW, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001. 103:532–537.
25. Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA. 2004. 292:1845–1852.
26. Sztriha LK, Vörös E, Sas K, Szentgyörgyi R, Pócsik A, Barzó P, et al. Favorable early outcome of carotid artery stenting without protection devices. Stroke. 2004. 35:2862–2866.
27. Theiss W, Hermanek P, Mathias K, Ahmadi R, Heuser L, Hoffmann FJ, et al. Pro-CAS: a prospective registry of carotid angioplasty and stenting. Stroke. 2004. 35:2134–2139.
28. Timaran CH. Clinical predictors of transient ischemic attack, stroke, or death within 30 days of carotid angioplasty and stenting. Perspect Vasc Surg Endovasc Ther. 2005. 17:384–385.
29. Wholey MH, Al-Mubarek N, Wholey MH. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003. 60:259–266.
30. Hofmann R, Niessner A, Kypta A, Steinwender C, Kammler J, Kerschner K, et al. Risk score for peri-interventional complications of carotid artery stenting. Stroke. 2006. 37:2557–2561.
31. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001. 357:1729–1737.
32. Roh HG, Byun HS, Ryoo JW, Na DG, Moon WJ, Lee BB, et al. Prospective analysis of cerebral infarction after carotid endarterectomy and carotid artery stent placement by using diffusion-weighted imaging. AJNR Am J Neuroradiol. 2005. 26:376–384.
33. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004. 351:1493–1501.
34. Ouriel K, Wholey MH, Fayad P, Katzen BT, Whitlow P, Frentzko M, et al. Feasibility trial of carotid stenting with and without an embolus protection device. J Endovasc Ther. 2005. 12:525–537.
35. Carlino M, De Gregorio J, Di Mario C, Anzuini A, Airoldi F, Albiero R, et al. Prevention of distal embolization during saphenous vein graft lesion angioplasty. Experience with a new temporary occlusion and aspiration system. Circulation. 1999. 99:3221–3223.
36. Theron J, Courtheoux P, Alachkar F, Bouvard G, Maiza D. New triple coaxial catheter system for carotid angioplasty with cerebral protection. AJNR Am J Neuroradiol. 1990. 11:869–874.
37. Henry M, Polydorou A, Henry I, Polydorou I, Hugel IM, Anagnostopoulou S. Angioplasty and stenting of extracranial vertebral artery stenosis. Int Angiol. 2005. 24:311–324.
38. Bogousslavsky J, Regli F, Hungerbuhler JP, Chrzanowski R. Transient ischemic attacks and external carotid artery. A retrospective study of 23 patients with an occlusion of the internal carotid artery. Stroke. 1981. 12:627–630.
39. Mames RN, Snady-McCoy L, Guy J. Central retinal and posterior ciliary artery occlusion after particle embolization of the external carotid artery system. Ophthalmology. 1991. 98:527–531.
40. Ohki T, Parodi J, Veith FJ, Bates M, Bade M, Chang D, et al. Efficacy of a proximal occlusion catheter with reversal of flow in the prevention of embolic events during carotid artery stenting: an experimental analysis. J Vasc Surg. 2001. 33:504–509.
41. Zahn R, Ischinger T, Mark B, Gass S, Zeymer U, Schmalz W, et al. Embolic protection devices for carotid artery stenting: is there a difference between filter and distal occlusive devices? J Am Coll Cardiol. 2005. 45:1769–1774.
42. Müller-Hülsbeck S, Jahnke T, Liess C, Glass C, Paulsen F, Grimm J, et al. In vitro comparison of four cerebral protection filters for preventing human plaque embolization during carotid interventions. J Endovasc Ther. 2002. 9:793–802.
43. Al-Mubarak N, Colombo A, Gaines PA, Iyer SS, Corvaja N, Cleveland TJ, et al. Multicenter evaluation of carotid artery stenting with a filter protection system. J Am Coll Cardiol. 2002. 39:841–846.
44. Angelini A, Reimers B, Della Barbera M, Sacca S, Pasquetto G, Cernetti C, et al. Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke. 2002. 33:456–461.
45. Whitlow PL, Lylyk P, Londero H, Mendiz OA, Mathias K, Jaeger H, et al. Carotid artery stenting protected with an emboli containment system. Stroke. 2002. 33:1308–1314.
46. Cremonesi A, Manetti R, Setacci F, Setacci C, Castriota F. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003. 34:1936–1941.
47. Castellan L, Causin F, Danieli D, Perini S. Carotid stenting with filter protection. Correlation of ACT values with angiographic and histopathologic findings. J Neuroradiol. 2003. 30:103–108.
48. White CJ, Iyer SS, Hopkins LN, Katzen BT, Russell ME. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day results. Catheter Cardiovasc Interv. 2006. 67:503–512.
49. Hill MD, Morrish W, Soulez G, Nevelsteen A, Maleux G, Rogers C, et al. Multicenter evaluation of a self-expanding carotid stent system with distal protection in the treatment of carotid stenosis. AJNR Am J Neuroradiol. 2006. 27:759–765.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download