Abstract
Breast cancer developing from a surgical scar is rare; this type of malignancy has been reported in only 12 cases to date. Herein, we report on a 52-year-old female who developed infiltrating ductal carcinoma in a surgical scar following excision of a benign mass. Two years previously, the patient underwent surgery and radiotherapy for invasive ductal carcinoma of the contralateral breast. The initial appearance of the scar was similar to fat necrosis; it was observed to be progressively shrinking on follow-up sonography. On the two year follow-up ultrasound, the appearance changed, an angular margin and vascularity at the periphery of the scar were noted. A biopsy and subsequent excision of the scar were performed; the diagnosis of infiltrating ductal carcinoma of the scar was confirmed.
Burns and chronic inflammation are well-known circumstances in which malignant transformation may occur during wound healing (1-3). There are many reports of malignancies developing from a burn scar (1-3); however, there are only a few reports of malignancy arising from chronic inflammation or from the skin of a surgical scar following excision of a benign mass, in any part of the body (1, 2, 4-6). There are only twelve reported cases of breast cancer developing from a surgical scar (7), moreover, there have been no reports in the last 30 years. This case is a recent example of breast cancer developing in a surgical scar that showed subtle changes on sequential sonograms.
In June 2003, a 50-year-old woman presented with a palpable mass in the subareolar region of her right breast with erythematous skin changes. She underwent mammography and sonography. A 2.5-cm diameter tumor with an irregular shape and high density on mammography was identified in the right breast, and a nonpalpable 1.2 cm irregular isoechogenic mass with an angular margin (Fig. 1A) was found in the left breast on sonography. A core biopsy revealed that the mass in the right breast was an infiltrating ductal carcinoma, and the mass in the left breast was an intraductal papilloma. The patient subsequently underwent five cycles of neoadjuvant chemotherapy with the adriamycin and taxol regimen followed by a right modified radical mastectomy and a right axillary node dissection in November 2003 (TNM staging: T2N3aM0). Excision of the mass in the left breast was performed under ultrasound guidance. Histopathology revealed an intraductal papilloma (Fig. 1B). The patient underwent, an additional, three cycles of adjuvant chemotherapy with the taxol regimen and radiation therapy to the right chest wall region.
At the six month follow-up sonography of the left breast showed architectural distortion and a mixed echogenic mass 1.5 cm in diameter at the inner portion of the left breast, with an internal anechoic cystic portion suggestive of fat necrosis (Fig. 1C). The patient underwent two additional ultrasounds during a 12-month period; the mixed echogenic mass appeared to be shrinking from 15 mm to 10 mm in diameter (Fig. 1D). A mammogram along with a second follow-up ultrasound was unremarkable. On the fourth follow-up ultrasound, performed in October 2005, the superficial portion of the mass showed a change in the appearance of the mass, which now had an angular margin with penetrating vascularity on Doppler imaging (Figs. 1E, F). Coned magnification views, of the left subareolar region, showed a 4 mm mass in the area corresponding to the sonographic abnormality (Fig. 1G). Abnormal findings were not identified by physical examination. A sonographically guided 14-gauge core needle biopsy yielded infiltrating ductal carcinoma. A lumpectomy confirmed the focal presence of infiltrating ductal carcinoma (maximal dimension: 4 mm), associated with marked fibrosis and foreign body reaction including multinuclear giant cells (Fig. 1H).
Freund et al. proposed several criteria for identification of breast cancer originating from surgical scars (7): 1) performance of a prior surgery in the affected breast area for an unrelated condition; 2) integrity of the breast prior to surgery; 3) complete healing of the surgical wound in the breast; 4) correspondence between the site of the tumor and the site of the surgical scar; and 5) reasonable time interval between surgery and appearance of the tumor. Our case fulfilled all five of these criteria. The subsequent tumor site corresponded exactly to the site of the surgical scar. We requested the pathologist to review again the previous specimen obtained from the excisional biopsy of the original mass from the left breast. The pathologist obtained additional levels from the original block, but nevertheless, again reported that these histopathological findings revealed only an intraductal papilloma.
Freund et al. did not define a specific time interval between surgery and the appearance of a newly developed cancer; the time interval in the Freund report ranged from one to 30 years between surgery and the appearance of breast cancer. In our case, the time interval between surgery and appearance of the tumor was two years. We suggest that a two year interval is sufficient time interval for the tumor to be regarded as a newly developed lesion. In the literature we reviewed, the twelve cases in which breast cancer developed at the site of old surgical scars, six cases had undergone breast biopsies, three drainage of a breast abscess and the remaining three had undergone thoracotomy that involved the breast area (7). However, none of these case reports demonstrated the sequential changes on imaging as the scar progressed to breast cancer. In our case, we were able to capture serial morphologic changes and observe the appearance of a breast cancer as it developed from a surgical scar.
The pathogenesis of carcinogenesis within scar tissue is not clearly understood. Several theories have been postulated. Scar tissue is poorly vascularized because dense fibrosis obliterates many blood vessels. This facilitates the development of ulceration and other ischemic conditions. The thin and delicate scar tissue is also more friable and susceptible to minor trauma (8). Avascular scar tissue tends to heal slowly and has a decreased resistance to infection. In addition, scar tissue undergoes a repeated repair processes, during which dysplasia may arise (1, 9). Moreover, because there are no lymphatic channels in scar tissue, a tumor may grow in isolation, unrestricted by immunologic resistance (9, 10). Thus, scar tissue may not only initiate malignant transformation, but also may provide an immunologically vulnerable site for growth. Other factors have also been suggested to be important including the implantation of living cells into the dermis as well as local environmental changes and trauma accompanied by potential carcinogenic agents (11). Both scar tissue and minor trauma appear to play a major role in malignant transformation, with a possible synergetic effect in this group of patients.
In our case, the patient also had an increased risk for breast cancer due to a history of breast cancer in the contralateral breast and a previous surgical biopsy in the ipsilateral breast, which revealed a papilloma. The combination of these factors appears to have contributed to the development of breast cancer. The fat necrosis-like lesions may have promoted a repeated repair process with inflammatory changes that included infiltrates of histiocytes and lymphocytes (12). Minor trauma to the surgical scar may also have been carcinogenic (11).
The imaging findings of this case were subtle but the mass that had been shrinking developed an angular margin in one region. These findings, along with the penetrating vascularity revealed on Doppler, prompted us to proceed to a biopsy of the lesion. It was of critical importance to compare the size and the shape of the lesion with previous ultrasounds in order to exclude malignancy in any iatrogenically altered lesion. Based on this case, we conclude that whenever a subtle change is detected, at a surgical site, a biopsy should be performed.
Figures and Tables
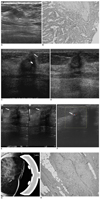 | Fig. 1
A. Sonography from June 2003 shows a 12 mm irregular mass (calipers) at the medial portion of the left breast.
B. The histopathologic findings from the left breast surgical specimen shows an intraductal papilloma (Hematoxylin & Eosin staining, ×100).
C. Follow-up sonography, from March 2004, shows an irregular mass with internal heterogeneous hyperechogenicity. An anechoic portion (thick arrow), suggestive of fat necrosis, was noted, and the mass abutted the thickened skin (thin arrow) from the previous excision.
D. The third follow-up sonogram from March 2005, demonstrates that the irregular mass decreased in size, from 15 mm to 10 mm.
E. The fourth follow-up sonography from September 2005, showing that the mass had developed a new angular margin (arrows) in one portion (right split-screen image: transverse view, left split-screen image: longitudinal view).
F. On Doppler ultrasound, penetrating vascularity (arrow) was detected.
G. On compression mammography of the craniocaudal view, a 4 mm mass (arrow) is seen just beneath the skin scar.
H. On tissue confirmation, the surgical specimen demonstrates a 4 mm infiltrating ductal carcinoma (arrows) in the peripheral portion of the
excised specimen with marked fibrosis and foreign body reaction in the majority of the specimen (Hematoxylin & Eosin staining, ×100).
|
References
1. Bowers RF, Young JM. Carcinoma arising in scars, osteomyelitis, and fistulae. Arch Surg. 1960. 80:564–570.
2. Engler HS, Fernandez A, Bliven FE, Moretz WH. Cancer arising in scars of old burns and in chronic osteomyelitis, ulcers, and drainage sites. Surgery. 1964. 55:654–664.
3. Kowal-Vern A, Criswell BK. Burn scar neoplasms: a literature review and statistical analysis. Burns. 2005. 31:403–413.
4. Ishida GM, Motoyama T, Watanabe T, Emura I. Clear cell carcinoma arising in a cesarean section scar. Report of a case with fine needle aspiration cytology. Acta Cytol. 2003. 47:1095–1098.
5. Lau YS, Banwell PE, Pay AD. Basal cell carcinoma arising in scars: late presentation 16 years after a midline sternotomy. Plast Reconstr Surg. 2004. 113:1297–1299.
6. Warren SB, Jim DA. Cutaneous neoplasm arising from total knee replacement incision in the early postoperative period. J Arthroplasty. 2004. 19:235–237.
7. Freund H, Biran S, Laufer N, Eyal Z. Breast cancer arising in surgical scars. J Surg Oncol. 1976. 8:477–480.
8. Treves N, Pack GT. The development of cancer in burn scars. Surg Gynecol Obstet. 1930. 51:749.
9. Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin's ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976. 57:66–69.
10. Arons MS, Rodin AE, Lynch JB, Lewis SR, Blocker TG Jr. Scar tissue carcinoma: II. An experimental study with special reference to burn scar carcinoma. Ann Surg. 1966. 163:445–460.
11. Neuman Z, Ben-Hur N, Shulman J. Trauma and skin cancer. Implantation of epidermal elements and possible cause. Plast Reconstr Surg. 1963. 32:649–656.
12. Baillie M, Mok PM. Fat necrosis in the breast: review of the mammographic and ultrasound features, and a strategy for management. Australas Radiol. 2004. 48:288–295.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download