Abstract
A 52-year-old male with right carotid body tumor underwent direct percutaneous glue (n-butylcyanoacrylate [NBCA]) embolization. Several hours later, he developed left hemiparesis from embolization of the polymerized glue cast. Migration of glue during percutaneous tumor embolization is presumed to occur only in the liquid state, which may lead to stroke or cranial nerve deficits. To the best of our knowledge, this is the first report of delayed glue embolization from a treated hypervascular tumor of the head and neck.
Direct percutaneous embolization of hypervascular tumors results in more effective preoperative devascularization. Migration of glue is a well-known complication of direct glue injection and it may lead to stroke or cranial nerve deficits. We report here on a case of carotid body tumor in a 52-year-old man; the tumor was mainly embolized by percutaneous injection of 50% glue and this was supported with balloon protection of the internal carotid artery. Thirteen hours later, he developed hemiparesis from delayed migration of glue. The possible mechanisms of this migration are discussed and preventive measures are suggested.
Preoperative embolization of hypervascular tumors of the head and neck, including carotid body tumor, is often performed to decrease the amount of blood loss during surgery (1-3). Devascularization is mainly performed with particulate agents and by employing the transarterial route. More effective embolization may be achieved by performing percutaneous direct embolization of hypervascular tumors with liquid embolic agents (1, 2, 4-8). Even though there are few reports available on direct embolization, complications from glue migration have been reported, and this mainly happens during the procedure when the glue is in a liquid state (9). We report here on a case of delayed migration of polymerized glue (n-butyl-2-cyanoacrylate [NBCA]), many hours after the procedure, into the intracranial circulation and the final result was stroke.
A 52-year-old male presented with a swelling of the right side of the neck of four months duration. The clinical examination was unremarkable except for the swelling. An MRI of the head and neck showed features that were typical of carotid body tumor. The routine laboratory investigations were within normal limits. The urinary vanilyl mandelic acid (VMA) level was 4.5 mg/day. The high vascularity of the tumor, along with features of arteriovenous shunting, was evident on the angiography (Figs. 1A-C). The tumor was supplied by feeders from the ipsilateral external carotid artery (ECA), internal carotid artery (ICA) and vertebral artery (VA). Preoperative embolization was performed under local anesthesia. Bilateral common femoral punctures were performed and 7 Fr vascular sheaths (Avanti+, Cordis, Miami Lakes, FL) were placed. One femoral access was used for balloon occlusion of the right carotid while the second access was used for performing controlled angiography. Since transarterial embolization through the right ascending pharyngeal artery failed to decrease the tumor's vascularity and the feeders were small in size and multiple in number, a decision was made to inject glue percutaneously into the tumor. Since there was a direct feeder from the right cervical ICA (Fig. 1B), a 6 Fr Berman angiographic balloon catheter (Arrow International, PA) was used to occlude the ICA across the origin of the feeder (Fig. 1C) with injection of glue. 50% glue was carefully injected under fluoroscopy. Care was taken to avoid any glue migration into the arterial or venous side. Glue was injected at three separate sites and more than 90% of the tumor was finally devascularized (Figs. 1D-F). The intracranial circulation was intact, the same as was seen on the preprocedure angiogram (Figs. 2A, B). Neurologically, the patient was intact at the end of the procedure. Care was taken to avoid palpation of the tumor site in the postoperative period. However, approximately 12 hours after the procedure, he developed left-sided hemiparesis. A brain CT scan was performed immediately and this showed multiple small fragments of glue located intracranially with non-hemorrhagic infarction of the right cerebral hemisphere (Fig. 2C). He improved with medical management. Six months later, he regained full power in the affected limbs. Digital subtraction angiography performed at this time showed partial recanalization of the tumor vascular bed. There was absence of any filling of the rolandic and parietal branches of the right middle cerebral artery (Fig. 2D), which was suggestive of persistent occlusion by the migrated glue.
Paragangliomas of the carotid bifurcation (carotid body tumors) are characterized by high vascularity and arteriovenous shunting (3, 4). Preoperative embolization is often performed to reduce the vascularity of these tumors so as to avoid any significant blood loss during surgery (4). Embolization of a carotid body tumor and other hypervascular tumors of the head and neck is usually performed through the transarterial route with using particulate agents (1-3). Although the ascending pharyngeal artery is considered to be the main feeder to a carotid body tumor, this type of tumor often derives its blood supply from multiple feeders of the ECA. Each of these feeders seems to supply individual compartments of a multicompartmental vascular bed of the tumor (3). The tortuous course and smaller caliber of some of the feeders, the proximity of the feeders to the carotid bifurcation, the tortuous carotids and the presence of carotid bifurcation atherosclerotic plaques may prevent effective transarterial embolization of carotid body tumors (4, 6). Further recruitment of a blood supply from the internal carotid artery and vertebral artery may preclude embolization through the transarterial route (1, 2).
Direct embolization provides an easy access to a hypervascular tumor with a higher chance of achieving complete devascularization (1, 2, 4). Casasco et al. were the first to use this technique to devascularize tumors of the head and neck (1). Direct embolization is typically carried out with NBCA (1, 2). While percutaneous tumor embolization offers an easy, safe access in the majority of the cases, it may occasionally lead to complications, the most serious being migration of glue into the intracranial circulation (1, 2, 4, 9). Migration of glue into the intracranial circulation may occur through an ECA-ICA communication, or it may occur in a retrograde fashion from the ECA into the ICA or through the branches arising from the internal carotid arteries or vertebral arteries. In a series of 65 cases of hypervascular tumors of the head and neck that were treated with glue, Casasco et al. had two complications related to migration of glue into the arterial side during embolization of juvenile nasopharyngeal angiofibroma (9). In one case, glue embolized to the middle cerebral artery and in the other it embolized to the ophthalmic artery. In both cases, migration of glue occurred towards the end of the procedure when the glue was being injected. In order to prevent this complication the authors emphasized performing an angiogram, as well as a parenchymogram, in at least two planes prior to embolization. Further, they recommended using a higher concentration of glue in the range of 50% to promote faster polymerization (9). Also, a non-detachable occlusal balloon may help prevent glue migration during embolization of carotid body tumors and juvenile angiofibromas. Using an occlusal balloon is recommended whenever there is a direct supply from the internal carotid artery or the vertebral artery (9).
We ensured that no glue migrated during the procedure by using 50% glue, as well as occlusal balloon, to occlude the ICA across the origin of the feeder from the ICA. Further, the occlusal balloon was kept inflated for at least 10 minutes after injecting glue into each sector of the tumor to allow complete polymerization. Controlled injection of glue ensured that no glue migrated during the procedure and consequently, we could observe filling of all the branches of the right anterior and middle cerebral arteries, as well as the ophthalmic artery. The intact neurological status of the patient for several hours after the procedure further ruled out migration of glue during the procedure. In the absence of a demonstrable glue cast in the ECA branches at the base of the skull, it is unlikely that the glue migrated through a dangerous ECA-ICA communication in our patient. It is highly likely that a polymerized glue cast in the tumor must have got fragmented several hours later and then some of it embolized into the intracranial circulation during the patient's postoperative period.
Migration of glue is presumed to happen only when the glue is in its liquid state during injection (1). Occurrence of stroke several hours later in our case illustrates that even polymerized glue may embolize into the intracranial circulation.
Figures and Tables
Fig. 1
A. Right carotid injection in the lateral view shows the hypervascular tumor with posterior displacement of the internal carotid artery.
B. Oblique view of right carotid injection with balloon occlusion of the external carotid artery shows the feeder from the internal carotid artery.
C. Balloon occlusion of the internal carotid artery with common carotid injection shows the residual vascular supply from the external carotid artery following embolization of the ascending pharyngeal artery. Note the tip of the needle for direct glue injection is well away from the internal carotid artery (arrow).
D. Glue cast of the tumor after final embolization.
E. Right common carotid injection in the lateral view at the end of the procedure shows near total devascularization of the tumor. A residual small area of tumor blush is seen posteriorly (arrow). Note the patent internal carotid artery flow. The overlying glue cast is mimicking artifactual filling defects in the internal carotid artery.
F. Right common carotid injection in the oblique view clearly shows the internal carotid artery lumen is separate from the glue cast (arrow).
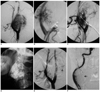
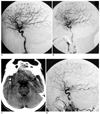
Fig. 2
A. Preprocedure right carotid injection in the lateral view shows the normal intracranial circulation.
B. Immediate post procedure right carotid injection in the lateral view shows the preserved flow in all the cortical branches of the middle cerebral artery, including the rolandic and parietal branches.
C. The non-enhanced CT scan of the brain at the level of the Circle of Willis shows the dense glue in the right sylvian fissure close to the location of the M2 segment (arrow).
D. Right carotid lateral view six months later shows non-visualization of the rolandic and parietal branches of the middle cerebral artery, and this is suggestive of persistent occlusion.
References
1. Casasco A, Herbreteau D, Houdart E, George B, Tran Ba Huy P, Deffresne D, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. AJNR Am J Neuroradiol. 1994. 15:1233–1239.
2. George B, Casasco A, Deffrennes D, Houdart E. Intratumoral embolization of intracranial and extracranial tumors: technical note. Neurosurgery. 1994. 35:771–773.
3. Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986. 7:943–952.
4. Abud DG, Mounayer C, Benndorf G, Piotin M, Spelle L, Moret J. Intratumoral injection of cyanoacrylate glue in head and neck paragangliomas. AJNR Am J Neuroradiol. 2004. 25:1457–1462.
5. Horowitz M, Whisnant RE, Jungreis C, Snyderman C, Levy EI, Kassam A. Temporary balloon occlusion and ethanol injection for preoperative embolization of carotid-body tumor. Ear Nose Throat J. 2002. 81:536–538. 540542 passim
6. Lonser RR, Heiss JD, Oldfield EH. Tumor devascularization by intratumoral ethanol injection during surgery. Technical note. J Neurosurg. 1998. 88:923–924.
7. Harman M, Etlik O, Unal O. Direct percutaneous embolization of a carotid body tumor with n-butyl cyanoacrylate: an alternative method to endovascular embolization. Acta Radiol. 2004. 45:646–648.
8. Liang Y, Wang D, Huang W, Ling F, Liu Y, Lu F. Direct intratumoral embolization of hypervascular tumors of the head and neck. Chin Med J. 2003. 116:616–619.
9. Casasco A, Houdart E, Biondi A, Jhaveri HS, Herbreteau D, Aymard A, et al. Major complications of percutaneous embolization of skull-base tumors. AJNR Am J Neuroradiol. 1999. 20:179–181.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download