Abstract
Traumatic neuroma is a well-known disorder that occurs after trauma or surgery involving the peripheral nerve and develops from a nonneoplastic proliferation of the proximal end of a severed, partially transected, or injured nerve. We present a case of traumatic neuroma around the celiac trunk after gastrectomy in a 56-year-old man, which was confirmed by pathology. CT demonstrated the presence of a lobulated, homogeneous, hypoattenuating mass around the celiac trunk, mimicking a nodal metastasis.
Traumatic neuroma is a well-known disorder that occurs after trauma or surgery involving the peripheral nerve and develops from a nonneoplastic proliferation of the proximal end of a severed, partially transected, or injured nerve (1, 2). However, in the abdomen, traumatic neuromas have been sporadically reported to occur in the bile duct (3-7). We present here a case of traumatic neuroma around the celiac trunk after gastrectomy that mimicks a nodal metastasis.
A 56-year-old man presented with a lobulated mass around the celiac trunk. This patient had undergone a distal gastrectomy with gastroduodenostomy for early gastric cancer nine years previously and a subtotal gastrectomy with gastrojejunostomy for recurred gastric cancer in the remnant stomach five months previously. According to the 5th American Joint Committee on Cancer (AJCC) TNM classification, the recurred gastric cancer was a stage II lesion (T3 N0 M0) based on the pathology (Fig. 1A). On the present admission, the patient showed no apparent discomfort and a physical examination was normal. The laboratory tests showed no abnormal findings. The carcinoembryonic antigen level was 1.6 ng/mL (< 5.0 ng/mL) and the serum carbohydrate antigen 19-9 level was 5.7 U/mL (< 39 U/mL).
A three-phase CT scan showed a lobulated, mildly enhanced, homogeneous, hypoattenuating mass just distal to the celiac trunk (Figs. 1B-E). This mass encased the common hepatic artery, splenic artery, proper hepatic artery, and the gastroduodenal artery (Figs. 1B, C). However, CT angiography showed no luminal narrowing or irregularities of these arteries (Fig. 1F). A fluorodeoxyglucose (FDG)-positron emission tomographic (PET) scan showed no increased uptake in the celiac region (mean standardized uptake value of 3.2) (Fig. 1G). Because it was difficult to distinguish a benign mass from a nodal metastasis, a decision was made to perform surgery.
During surgery, an ill-defined, irregular, pale tan, firm mass was seen around the celiac trunk. A frozen section revealed the presence of fibrotic connective tissue without malignant cells, and the proliferation of neural tissue. The tumor mass was resected in several pieces (Fig. 1H). The size of the mass was 3.0 × 3.5 × 1.0 cm in aggregates. A histologic examination showed proliferation of neural tissue and fibrotic change, but there was no evidence of definite malignant cells. The celiac mass consisted of small and large proliferating fascicles of nerve in a background of collagen and fat tissue (Fig. 1I, J). These findings were compatible with the presence of a traumatic neuroma.
On a follow up CT scan performed seven months later, there was no evidence of a soft tissue mass in the celiac region.
Traumatic or amputation neuroma is a well-known disorder that occurs 1-12 months after trauma or surgery involving the peripheral nerve. Rather than representing a neoplasm, neuroma represents a reactive hyperplasia of nerve tissue and usually occurs at the proximal end of a severed nerve (2).
In the abdomen, neuromas have been reported to occur in the bile duct after cholecystectomy (3, 4), bile duct surgery (5), orthotopic liver transplantation (6), and blunt abdominal trauma (7). The neuromas occur as most of the common bile duct is surrounded by a delicate net of sympathetic and parasympathetic nerve fibers that are derived from the celiac plexus. Most patients have no symptoms, but some patients have upper abdominal pain and jaundice. Treatment of this disorder is unnecessary unless the patient has symptoms.
The celiac plexus, surrounding the root of the celiac arterial trunk, are nerve networks consisting of both sympathetic and parasympathetic fibers. Injury to celiac plexus during lymph node dissection for gastric surgery is possible.
Arterial invasion can be suggested with high specificity when either arterial embedment, tumor involvement exceeding one-half of the circumference of the vessel, vessel wall irregularities, or vessel caliber stenosis is present (8). In the present case, the mass encircled the whole circumference of multiple vessels, but in any of the encircled vessels, wall irregularity or vessel caliber stenosis was not seen with CT angiography. This finding may be unusual to a malignant tumor.
The tumor was homogeneous and hypovascular in arterial, portal, and equilibrium phases of an enhanced CT scan. There was also no increased uptake of FDG on the FDG-PET scan, suggestive of the presence of a benign hypovascular mass.
FDG is not a cancer-specific agent. False positive findings have been reported in cases of active inflammation and infection, and malignant tumors with low metabolic activity or tumors smaller than 1.0 cm in diameter often show false negative results (9). In detecting local lymph node metastasis from stomach cancer, the sensitivity of PET was lower than that of CT, and the specificity was higher. However, the overall accuracy of PET was not significantly different from that of CT in detecting both local (63% vs. 75%) and distant (95% vs. 89%) lymph node metastasis (10).
Our initial impression suggested that the mass was a conglomerated nodal metastasis or postoperative granuloma. A preoperative diagnosis of traumatic neuroma is difficult to make, despite all modern imaging techniques, and in most cases, a final diagnosis is made at surgery.
In conclusion, the imaging finding of traumatic neuroma around the celiac trunk was a homogeneous hypovascular mass without narrowing or irregularity of encased arteries and without increased uptake on PET-CT. Although from a clinical standpoint, establishing an accurate preoperative diagnosis is difficult to perform, the presence of a traumatic neuroma should be included in the differential diagnosis of a mass around the celiac trunk in a patient that has undergone celiac nodal dissection.
Figures and Tables
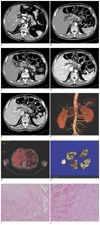
Fig. 1
A 56-year-old man with traumatic neuroma around the celiac trunk.
A. A contrast-enhanced abdominal CT scan in the arterial phase showed no mass present around the celiac trunk 5 months previously.
B, C. Contrast-enhanced abdominal CT scans in the arterial phase show a lobulated hypoattenuating soft tissue mass in the celiac region encasing the common hepatic artery, splenic artery, gastroduodenal artery, and the proper hepatic artery.
D, E. Contrast-enhanced abdominal CT scans in the portal phase (D) and equilibrium phase (E) show a mild homogeneous enhancement of this mass.
F. Volume-rendering CT angiography shows that the celiac trunk and its tributaries are patent without vessel wall irregularities, or vessel caliber stenosis.
G. Fluorodeoxyglucose (FDG)-positron emission tomography (PET) image shows no increased uptake around the celiac trunk with a mean standardized uptake value of 3.2.
H. The resected celiac mass is composed of several pieces of irregular pale tan to yellow firm tissue, measuring 3.5 × 3.0 × 1.0 cm in aggregates.
I, J. Microscopically, the celiac mass consists of small (arrows) and large (asterisk) proliferating fascicles of nerve in a background of collagen and fat tissue (Hematoxylin & Eosin staining, × 100). The celiac mass is composed of a haphazard proliferation of nerve fascicles, including axons with their investitures of myelin, Schwann cells, and fibroblasts (Hematoxylin & Eosin staining, × 200).
References
1. Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999. 19:1253–1280.
2. Yabuuchi H, Kuroiwa T, Fukuya T, Tomita K, Hachitanda Y. Traumatic neuroma and recurrent lymphadenopathy after neck dissection: comparison of radiologic features. Radiology. 2004. 233:523–529.
3. Nagata Y, Tomioka T, Chiba K, Kanematsu T. Traumatic neuroma of the common hepatic duct after laparoscopic cholecystectomy. Am J Gastroenterol. 1995. 90:1887–1888.
4. Shimura K, Tamada K, Asada M, Watabiki N, Wada I, Tanaka N, et al. Intraductal ultrasonography of traumatic neuroma of the bile duct. Abdom Imaging. 2001. 26:632–634.
5. van Gulik TM, Brumelkamp WH, Lygidakis NJ. Traumatic neuroma giving rise to biliary obstruction after reconstructive surgery for iatrogenic lesions of biliary tract-a report of three cases. Hepatogastroenterology. 1989. 36:255–257.
6. Mentha G, Rubbia-Brandt L, Orci L, Becker C, Giostra E, Majno P, et al. Traumatic neuroma with biliary duct obstruction after orthotopic liver transplantation. Transplantation. 1999. 67:177–179.
7. Katsinelos P, Dimiropoulos S, Galanis I, Tsolkas P, Paroutoglu G, Arvaniti M, et al. Biliary stricture due to neuroma after an innocent blunt abdominal trauma. Surg Endosc. 2002. 16:1494.
8. Li H, Zeng MS, Zhou KR, Jin DY, Lou WH. Pancreatic adenocarcinoma: the different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomogr. 2005. 29:170–175.
9. Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006. 7:57–69.
10. Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005. 103:2383–2390.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download