Abstract
Objective
We wanted to assess the relationship between pain and the prostate volume during transrectal ultrasound (TRUS) guided biopsy.
Materials and Methods
Between July and September 2006, 71 patients scheduled for TRUS biopsy of the prostate were considered for inclusion to this study. These patients underwent periprostatic neurovascular bundle block with lidocaine prior to biopsy. Pain was assessed using a Visual Analogue Scale (VAS) during periprostatic neurovascular bundle block (VAS 1), during biopsy (VAS 2), and 20 minutes after biopsy (VAS 3). The mean pain scores were analyzed in the large prostate group (prostate volume > 40 cc) and the small prostate group (prostate volume ≤ 40 cc). P values < 0.05 were considered significant.
Results
The mean prostate volume was 42.2 cc (standard deviation: 8.6). The mean pain scores of VAS 1, 2 and 3 were 4.70 ± 1.61, 3.15 ± 2.44 and 1.05 ± 1.51, respectively. In the large prostate group, the mean pains scores of VAS 1, 2 and 3 were 4.75 ± 1.76, 3.51 ± 2.76 and 1.29 ± 1.70, respectively, whereas in the small prostate group, the means pain scores were 4.66 ± 1.46, 2.77 ± 2.0, and 0.80 ± 1.26, respectively. Although there were no statistical differences of VAS 1, the larger prostate group revealed higher pain scores of VAS 2 and 3 compared with the small prostate group (p < 0.05).
The diagnosis of prostate cancer depends on obtaining histologically malignant tissue from the prostate gland by performing biopsy. Transrectal ultrasound (TRUS) guided biopsies of the prostate are the accepted gold standard for detecting prostate cancer. Even though a prostate biopsy is generally considered a minor procedure that is well tolerated by most patients, a few studies have reported that about 65 to 90% of patients complain of discomfort or pain (1, 2).
Several investigators have evaluated the relationship between pain during a TRUS guided prostate biopsy procedure and the prostate volume. Although the recently published findings have indicated that larger prostate volumes do not necessarily increase the pain associated with the procedure (3, 4), our clinical impression, based on experience, is that the prostate volume is certainly related to the patient's degree of pain during TRUS guided prostate biopsy.
The aim of this study was to assess the relationship between pain and the prostate volume during a TRUS guided prostate biopsy procedure.
This prospective study was approved by our institutional review board. All the patients gave us a signed, written informed consent form prior to performing TRUS guided biopsy.
During the three month period from July to September 2006, 71 patients who satisfied the following criteria were enrolled in this study: 1) patients with increased levels of prostate specific antigen (PSA) with or without an abnormal digital rectal examination; 2) patients with lesion suspected to be malignancy on TRUS with or without an abnormal digital rectal examination; 3) patients without a history of warfarin treatment or a bleeding tendency or allergy to lidocaine; 4) patients without a history of previous TRUS guided prostate biopsy. Two radiologists performed the prostate biopsies. All imaging was performed using a HDI 5000 ultrasound scanner (Philips, Bothell, WA) equipped with 9-4 MHz broadband curved array endocavitary transducer.
For all 71 patients, a total of 8 ml of 1% lidocaine was administered, at both basolateral aspects of the prostate, five minutes prior to the prostate biopsies, and a 22 gauge needle was used for this (SONORIJECT; TSK Laboratory, Japan) (Fig. 1).
The prostate volumes were evaluated using the prolate ellipse formula ( π/6 [transverse diameter X anterior-posterior diameter X cephalocaudal diameter]), which is the most commonly used method of determining prostate volumes.
After determining the prostate volume, a total of 12 systematic prostate cores, including six parasagitally targeted biopsies and six laterally targeted biopsies covering the base, mid zones and apexes, were obtained from all the patients with using an 18 gauge spring loaded biopsy gun (ACECUT; TSK Laboratory, Japan). The first biopsy site was the left apex; this was followed by biopsy at the left middle area, the base, the right apex, the right middle area and the base. All the patients were observed for at least one day after the procedure.
During procedures, the patients were asked to score their degree of pain with using a Visual Analogue Scale (VAS), 1) during the periprostatic neurovascular bundle block (VAS 1), 2) during the biopsy (VAS 2), and 3) 20 minutes after biopsy (VAS 3) (0; no pain, 10: unbearable pain). The VAS we used had a length of 0 to 10 cm, and the patients' assessments were converted into numeric scores.
We divided the patients into two groups: the large prostate group (prostate volume > 40 cc) and the small prostate group (prostate volume ≤ 40 cc).
Statistical analysis was performed using SPSS version 11.0 (SPSS, Chicago, IL). Levene's tests were used to assess the equality of variances between the patients in the two groups. Student t tests were performed for making comparison of age, PSA levels and the pain scores at each stage (VAS 1, 2 and 3) between the large prostate and small prostate groups, respectively, and Fishers' exact tests were used to assess the difference in the cancer prevalence between the two groups. P values < 0.05 were considered statistically significant.
The mean patient age, PSA level and prevalence of cancer were similar between the two groups (Table 1). The mean prostate volume was 42.2 cc (standard deviation: 8.6), and the mean total prostate volumes were 52.4 cc (range: 41.0 cc to 92.9 cc) in the large prostate group and 31.2 cc (range: 17.2 cc to 39.9 cc) in the small prostate group. None of the patients experienced major complications, including massive rectal bleeding or infection.
Table 2 shows the VAS pain scores and the statistical analysis of the VAS scores for comparing the large and small prostate groups. The mean VAS scores during periprostatic neurovascular bundle block, during biopsy and 20 minutes after biopsy were 4.70 ± 1.61, 3.15 ± 2.44, and 1.05 ± 1.51, respectively. In the large prostate group, the mean pain scores of VAS 1, 2 and 3 were 4.75 ± 1.76, 3.51 ± 2.76 and 1.29 ± 1.70, respectively, whereas in the small prostate group, the mean pain scores were 4.66 ± 1.46, 2.77 ± 2.03 and 0.80 ± 1.26, respectively. Although there was no statistical difference between the two groups during neurovascular bundle block (VAS 1), the patients with a large prostate felt more pain during biopsy (p = 0.045) and 20 minutes after biopsy (p = 0.049) (Table 2).
Transrectal ultrasound guided prostate biopsy is an essential diagnostic modality for making the preoperative diagnosis of patients with a suspected malignancy. Unfortunately, patients generally experience significant pain during the procedure. Irani et al. reported that a significant proportion of patients who underwent TRUS guided prostatic biopsy felt pain, and biopsy should be accompanied by some form of anesthesia (5). Moreover, because of the patient's fear of a potential diagnosis of cancer and also fear of using the anal route, and too the fact that the examined organ is part of the male sexual system, the pain experienced during TRUS guided prostate biopsy may induce vasovagal episodes in up to 5.3% of the men (3, 6-8).
Given the demand for an increasing number of biopsies, investigators are now showing an increased interest in the risk factors for experiencing pain during TRUS guided prostate biopsy. Identifying these risk factors is important in terms of selecting those subgroups of patients who may benefit from anesthesia or drugs. However, few of the risk factors for pain have been established. A younger age, anxiety, the number of cores taken and repeat biopsy appear to be the risk factors of a painful biopsy (3, 4, 9-13).
To the best of our knowledge, few studies have accessed the relationship between prostate volume and biopsy pain. According to recent reports, higher prostate volumes do not necessarily increase the pain associated with the procedure (3, 4).
Bastide et al. performed a prospective study to identify the risk factors of pain during prostate biopsy. According to their report, among the six factors they studied (age, prostate volume, number of cores, previous biopsy and location of the first core), only the first core location influenced the pain (3). However, their VAS was obtained only once by employing an immediate postbiopsy questionnaire.
In this study, we also divided the patients into two groups according to their prostate volume with a cutoff value of 40 cc, which was based on the criterion of a previous study by Bastide et al. (3).
However, the results of the present prospective comparative study, in which number of cores, the presence or absence of a previous history of biopsy and the location of the first core were controlled and the patients' mean age showed equal variances between the two groups, showed that patients with a larger prostate experienced more pain during the TRUS guided prostate biopsy. There was positive correlation between the prostate volume and pain with statistical significance during and 20 minutes after biopsy.
Pain associated with the prostate predominantly arises in the prostatic capsule or stroma; these are structures richly innervated with autonomic fibers. Innervation of the prostate is derived from the caudal roots of S2 to S5 and also from the sympathetic chain via the presacral and hypogastric neural plexuses. Nerve fibers from these nerve plexuses branch out in the prostatic plexus and then they travel with the prostatic vascular pedicles. It is believed that these posterolateral nerve fibers are the main nerve supply to the prostate (14-16). Issa et al. reported that pain is generated by direct contact between these nerves and the needle as it passes through the prostate (6).
Based on these considerations, some urologists have attempted periprostatic neurovascular bundle block before prostate biopsy, and many investigators have shown that local anesthesia is effective for reducing patient discomfort (14, 17-19).
We presume that a more sparsely distributed periprostatic neurovascular bundle and the reduced effectiveness of the locally injected lidocaine to the sparsely distributed nerve fibers in a larger prostate may contribute to the positive correlation found between prostate volumes and pain in our series, although any histological investigation about the difference in distribution of the periprostatic neurovascular bundle between large and small prostates has not been reported. There may be other contributing factors such as more severe anal pain or the pain from stimulation of the prostate is generalized by manipulation of probe during biopsy of the larger prostate. Further studies about the distributions of periprostatic neurovascular bundles in correlation with the prostate volume and the relation between the prostate volume and biopsy pain with using varying amounts of injected lidocaine are required.
During periprostatic neurovascular bundle block, no correlation was found between the prostate volume and pain. We believe that pain during periprostatic neurovascular bundle block is composed of pain associated with the rectal wall when the probe is inserted through the rectum and when the needle pierces the rectal wall, and it is not reasonable to assume there is significant correlation between prostate volume and pain during periprostatic neurovascular bundle block.
In the present study, periprostatic neurovascular bundle block was the most painful step. Although which step is the most painful during TRUS guided prostate biopsy has not been universally confirmed, investigators have recently reported the initial probe insertion with periprostatic lidocaine instillation is more painful than the biopsy itself when the biopsy is performed under periprostatic neurovascular bundle block (20, 21). The present data corroborates with the results of the previous studies. Even though periprostatic neurovascular bundle block generalizes the additional pain during procedure, it seems that periprostatic neurovascular bundle block is effective to reduce the overall pain during TRUS guided prostate biopsy (14, 17-19).
There are several limitations in our study. First, we did not consider some parameters, including the skill of the operator that may affect the severity of the pain. Second, although the study design was prospective, the way or attitude in which the investigator handled the questionnaire with interviewing patients may have induced some bias because the investigator knew the patients' prostate volume. So, a double blinded randomized study could show more objective results.
In conclusion, patients with larger prostate volumes were found to experience higher degrees of pain during and after TRUS guided prostate biopsy. This finding suggests that additional analgesic strategies may be necessary for performing TRUS guided prostate biopsies of larger prostates.
Figures and Tables
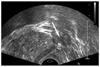 | Fig. 1Ultrasonographic image of the anesthetic injection site during periprostatic neurovascular bundle block. Arrow indicates the site of lidocaine injection (P; prostate). |
References
1. Clements R, Aideyan OU, Griffiths GJ, Peeling WB. Side effects and patient acceptability of transrectal biopsy of the prostate. Clin Radiol. 1993. 47:125–126.
2. Collins GN, Lloyd SN, Hehir M, McKelvie GB. Multiple transrectal ultrasound-guided prostatic biopsies-true morbidity and patient acceptance. Br J Urol. 1993. 71:460–463.
3. Bastide C, Lechevallier E, Eghazarian C, Ortega JC, Coulange C. Tolerance of pain during transrectal ultrasound-guided biopsy of the prostate: risk factors. Prostate Cancer Prostatic Dis. 2003. 6:239–241.
4. Rodriguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol. 1998. 160:2115–2120.
5. Irani J, Fournier F, Bon D, Gremmo E, Dore B, Aubert J. Patient tolerance of transrectal ultrasound-guided biopsy of the prostate. Br J Urol. 1997. 79:608–610.
6. Issa MM, Bux S, Chun T, Petros JA, Labadia AJ, Anastasia K, et al. A randomized prospective trial of intrarectal lidocaine for pain control during transrectal prostate biopsy: the Emory University experience. J Urol. 2000. 164:397–399.
7. Wu CL, Carter HB, Naqibuddin M, Fleisher LA. Effect of local anesthetics on patient recovery after transrectal biopsy. Urology. 2001. 57:925–929.
8. Zisman A, Leibovici D, Kleinmann J, Siegel YI, Lindner A. The impact of prostate biopsy on patient well-being: a prospective study of pain, anxiety and erectile dysfunction. J Urol. 2001. 165:445–454.
9. Aus G, Hermansson CG, Hugosson J, Pedersen KV. Transrectal ultrasound examination of the prostate: complications and acceptance by patients. Br J Urol. 1993. 71:457–459.
10. Presti JC Jr. Prostate biopsy: how many cores are enough? Urol Oncol. 2003. 21:135–140.
11. Naughton CK, Ornstein DK, Smith DS, Catalona WJ. Pain and morbidity of transrectal ultrasound guided prostate biopsy: a prospective randomized trial of 6 versus 12 cores. J Urol. 2000. 163:168–171.
12. Autorino R, De Sio M, Di Lorenzo G, Damiano R, Perdona S, Cindolo L, et al. How to decrease pain during transrectal ultrasound guided prostate biopsy: a look at the literature. J Urol. 2005. 174:2091–2097.
13. Leibovici D, Zisman A, Siegel YI, Sella A, Kleinmann J, Lindner A. Local anesthesia for prostate biopsy by periprostatic lidocaine injection: a double-blind placebo controlled study. J Urol. 2002. 167:563–565.
14. Nash PA, Bruce JE, Indudhara R, Shinohara K. Transrectal ultrasound guided prostatic nerve blockade eases systematic needle biopsy of the prostate. J Urol. 1996. 155:607–609.
15. Hollabaugh RS Jr, Dmochowski RR, Steiner MS. Neuroanatomy of the male rhabdosphincter. Urology. 1997. 49:426–434.
16. Hollabaugh RS Jr, Dmochowski RR, Kneib TG, Steiner MS. Preservation of putative continence nerves during radical retropubic prostatectomy leads to more rapid return of urinary continence. Urology. 1998. 51:960–967.
17. Soloway MS, Obek C. Periprostatic local anesthesia before ultrasound guided prostate biopsy. J Urol. 2000. 163:172–173.
18. Alavi AS, Soloway MS, Vaidya A, Lynne CM, Gheiler EL. Local anesthesia for ultrasound guided prostate biopsy a prospective randomized trial comparing 2 methods. J Urol. 2001. 166:1343–1345.
19. Stirling BN, Shockley KF, Carothers GG, Maatman TJ. Comparison of local anesthesia techniques during transrectal ultrasound-guided biopsies. Urology. 2002. 60:89–92.
20. Inal G, Yazici S, Adsan O, Ozturk B, Kosan M, Cetinkaya M. Effect of periprostatic nerve blockade before transrectal ultrasound-guided prostate biopsy on patient comfort: a randomized placebo controlled study. Int J Urol. 2004. 11:148–151.
21. Schostak M, Christoph F, Muller M, Heicappell R, Goessl G, Staehler M, et al. Optimizing local anesthesia during 10-core biopsy of the prostate. Urology. 2002. 60:253–257.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download