Abstract
Objective
We wanted to assess the need for surgical excising papillary lesions of the breast that were diagnosed upon sonographically guided 14-gauge core needle biopsy.
Materials and Methods
Sixty-nine women (age range: 25-74 years, mean age: 51.7 years) with 69 papillary lesions (4.9%) were diagnosed and followed after performing sonographically guided 14-gauge core needle biopsies. Surgical excision was performed for 44 (64%) of 69 papillary lesions, and 25 lesions were followed with imaging studies (range: 6-46 months, mean: 17.9 months). The histologic findings upon core biopsy were compared with the surgical, imaging and follow-up findings.
Results
Core needle biopsies of 69 lesions yielded tissue that was classified as benign for 43 lesions, atypical for 18 lesions and malignant for eight lesions. Of the 43 lesions that yielded benign papilloma upon core needle biopsy, one had intraductal papillary carcinoma found upon surgery. An immediate surgical biopsy was recommended for this lesion because of the imaging-histologic discordance. No additional carcinoma was found during the imaging follow-up. Surgical excision was performed for 17 atypical papillary lesions, and this revealed intraductal (n = 6) or invasive (n = 2) papillary carcinoma in 8 (47%) lesions. Of the seven intraductal papillary carcinomas, surgery revealed invasive papillary carcinoma in one (14%).
Papillary lesions of the breast account for less than 10% of all benign breast neoplasms that are biopsied, and they are 1-2% of all breast carcinomas (1-4). Papillary breast proliferations form a spectrum of lesions that are difficult to diagnose both with using the excision biopsy specimens and the diagnostic core biopsy specimens (5, 6). Surgical excision has been used to establish the diagnosis in most cases. However, the results of recent studies have suggested that percutaneous core biopsy with imaging concordance may be sufficient for the diagnosis and management of papillary lesions (7, 8). However, most of these studies generally used stereotactic guidance and the number of cases was relatively small.
The advantages of sonography as a guidance modality for percutaneous breast biopsy include the lack of ionization radiation, the use of non-dedicated equipment and the real-time needle visualization. For lesions amenable to either stereotactic or sonographically guided biopsy, sonographically guided biopsy is preferable in terms of patient comfort, the procedure time and cost (9, 10).
The purpose of our study was to assess the need for surgical excising papillary lesions of the breast that were diagnosed via sonographically guided 14-gauge core needle biopsy.
Between January 2001 and April 2005, sonographically guided 14-gauge core needle biopsies were performed on 1,566 consecutive lesions at our institution, and 76 (4.9%) of these were diagnosed as papillary lesions. Using an outcomes audit of our biopsy database, we retrospectively identified all the cases of papillary lesions, including papilloma, papillomatosis, atypical papilloma, noninvasive papillary carcinoma and invasive papillary carcinoma (6). Seven of the 76 cases were lost to follow-up and so they were excluded from this study. The remaining 69 papillary lesions in 69 women (age range: 25-74 years, mean age: 51.7 years) constituted our study population. Of the 69 patients, 29 (42%) presented with palpable mass, three (4%) with breast pain, nine (13%) with nipple discharge, 11 (16%) with screening mammographic abnormalities and 17 women (25%) with mammographic dense breast had sonographic abnormality. Surgical excision was performed for 44 (64%) of 69 papillary lesions. The remaining 25 (36%) lesions underwent imaging follow-up and the mean duration of follow-up was 17.9 months (range: 6-46 months).
All biopsies were guided using high-resolution sonography units with 10- or 12-MHz linear transducers (Voluson 730, Kretz, Austria; HDI 5000, Advanced Technology Laboratories, Bothell, WA) with the patient in the supine or supine oblique position. The biopsies were performed using a freehand technique with a 14-gauge automated needle (Bard Peripheral Technologies, Convinton, GA) and a spring-loaded biopsy gun (Pro-Mag 2.2, Manan Medical Products, Northbrook, IL). The biopsy procedures were performed by four fellows and three staff radiologists; the radiologists each had between 2-8 years of experience with breast imaging. The mean number of core samples obtained with the 14-gauge automated gun was five (range: 3-10).
The imaging findings for all lesions were retrospectively reviewed by two radiologists working in consensus. All the lesions were evaluated for size. The US appearances of the lesions were characterized according to the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) lexicon and the final assessment categories (11, 12).
The pathology results were prospectively compared with the relevant imaging findings to determine concordance or discordance of the biopsy results. Decisions were made by two radiologists working in consensus. Benign pathology was considered concordant if no imaging features that were highly suspicious for malignancy were present, accurate targeting of the needle was shown, adequate samples were obtained and the pathology results suggested a process known to manifest as a mass (7, 8). The findings were discordant when a BI-RADS category of 5 (highly suggestive of malignancy) was given to the lesion at imaging and the corresponding histologic finding was benign. If a lesion's imaging was determined to be concordant with benign pathology, then 6, 12 or 24 months of follow-up was recommended (Fig. 1). All the pathology results that revealed atypia, intraductal or invasive carcinoma received recommendations for surgical excision. Those benign lesions for which an operation was requested by the pathologist and radiologist, due to imaging-pathologic discordance, were also excised.
"Histologic underestimation" was defined as a lesion yielding atypical ductal hyperplasia (ADH) upon percutaneous biopsy and carcinoma upon surgery (ADH underestimation), or a lesion that yielded ductal carcinoma in situ (DCIS) upon core needle biopsy and invasive carcinoma upon surgery (DCIS underestimation) (13, 14). The underestimation rate of ADH or DCIS was determined by dividing the total number of lesions, for which excisional biopsy was performed, by the number of lesions that proved to be DCIS or invasive carcinoma upon excision. When repeat biopsy was performed, the time interval and the reason for the second biopsy were recorded. An immediate rebiopsy was considered when the repeat biopsy was performed before the first sonographic follow-up. A delayed rebiopsy was considered when the repeat biopsy was performed after sonographic follow-up. The repeat biopsy rate was determined by dividing the total number of core needle biopsies by the number of repeat biopsies. The false negative rate was determined by dividing the total number of carcinomas found upon subsequent biopsy by the number of those lesions that were previously benign upon core needle biopsy (15-17).
Forty-three benign papillomas (62%), 18 atypical papillomas (26%), seven intraductal papillary carcinomas (10%), and one invasive papillary carcinoma (2%) were diagnosed upon core needle biopsy. All the lesions with a histologic diagnosis of malignancy (n = 8) or atypia (n = 17), except one atypical papilloma, upon core needle biopsy were surgically excised. Of the 43 lesions that were diagnosed upon core needle biopsy as benign, 19 lesions underwent surgical excision. The histologic findings upon core needle biopsy and surgical excision are given in Tables 1 and 2.
At sonography, the mean size was 1.3 cm (range: 0.5-3.8 cm) for all the papillary lesions. These lesions manifested sonographically as hypoechoic solid masses in 40 patients, intracystic masses in three patients, complex masses with solid and cystic components in nine patients, and as a mass within a dilated duct in 17 patients. Six lesions were round, 10 were oval and 53 were irregular in shape. The margins were circumscribed in 16 lesions and noncircumscribed in 53 lesions. The final assessment of 69 lesions, based on the combined mammographic and sonographic findings, was category 3 (probably benign lesions) for nine masses (13%), category 4a (low suspicion lesions) for 47 masses (68%), category 4b or 4c (moderate suspicion lesions) for 12 masses (18%) and category 5 (highly suggestive of malignancy) for one mass (1%) (Table 2).
The imaging findings and the histopathologic findings for 42 of the 43 benign lesions (Fig. 1), for all 18 atypical lesions, and for all eight malignant lesions were concordant. There was discordance between the imaging findings and the histopathologic findings for one of the benign lesions upon core needle biopsy (Fig. 2). The mammograms and sonographic findings demonstrated a 1.6 cm irregular hypoechoic mass and it was assessed as category 5, that is, highly suggestive of malignancy. Subsequent surgical excision and histopathologic evaluation of this lesion revealed an intraductal papillary carcinoma. All but one of the atypical papillomas underwent excision. The surgical biopsy results revealed benign findings without atypia in two lesions (12%), atypical papilloma in seven lesions (41%), intraductal papillary carcinoma in six lesions (35%), and invasive papillary carcinoma in two lesions (12%) (Table 1). The ADH underestimation rate was 47% (8/17). Of the seven lesions yielding intraductal papillary carcinoma upon core needle biopsy, surgical excision revealed intraductal papillary carcinoma in five lesions (71%), invasive papillary carcinoma in one lesion (14%) and one atypia without carcinoma. Therefore, the DCIS underestimation rate was 14% (1/7).
Repeat biopsy was performed for 19 of the 43 patients with benign papillary lesions and for 17 of the 18 patients with atypical papillary lesions. For repeat biopsy, surgical excision was done in all cases and this was performed because of the patient's or physicians' concern (n = 18), imaging-histologic discordance (n = 1), and atypia findings (n = 17). All the repeat biopsies, except one, were performed immediately after the first core needle biopsy. Delayed repeat biopsy was performed for only one benign lesion because of the patient's concern. The repeat biopsy rate was 52% (36/69); the false negative rate was 6.3% (1/19).
Twenty-four (56%) of the 43 lesions that were diagnosed as benign were not surgically excised and they underwent imaging follow-up (range: 6-46 months; mean: 17.9 months). One atypical papilloma that was not surgically excised underwent 24 months of imaging follow-up. No additional carcinoma was detected during the follow-up period. All the lesions were stable (n = 20) or they had decreased in size (n = 5).
In our series, 4.9% (76/1,566) of the sonographically guided core needle biopsies performed at our institution revealed papillary lesions. This frequency is same as the 4.9% (36/734) by Liberman et al. (7) and it is higher than the 3.2% (34/1,077) by Mercado et al. (18). Our study included 17 (25%) sonographic abnormalities in patients with mammographic dense breast, while most of the cases in the other studies were detected by mammographic abnormalities. Lesions that are neither palpable nor mammographically visible can be detected on sonography during the evaluation of unrelated mammographic or clinical findings (10). Recent studies have reported that women with mammographically dense breasts have a considerably increased rate for detecting breast cancer when whole-breast sonography is performed (19-21).
The results of the previously published series, including our own data, are shown in Table 3. The outcomes of sonographically guided 14-gauge core needle biopsy in our study were not inferior to the stereotactic guided 14- and 11-gauge needle biopsies in terms of repeat biopsy and the false negative rate. In a study by Mercado et al. (18), an 11-gauge vacuum-assisted biopsy device was used to perform all the percutaneous biopsies. Previous studies have demonstrated that a larger tissue sample is obtained per core specimen with the 11-gauge stereotactic directional vacuum-assisted biopsy technique versus the 14-gauge core biopsy technique (14, 16). This provides for a larger lesion sample, which may help to more accurately identify lesions. Therefore, Mercado's outcomes would seem to be better than some of the other studies in terms of the repeat biopsy rate and the ADH underestimation rate.
Controversy still persists regarding the need for excision of papillomas that are diagnosed upon percutaneous breast biopsy (22, 23). In a recent study by Liberman et al. (23), cancer was found in five (14%) of the 35 lesions that yielded a benign, concordant diagnosis of papilloma upon percutaneous biopsy. Surgery also revealed six (17%) high-risk lesions, including atypical ductal hyperplasia (n = 3), radial scar (n = 2), and lobular carcinoma in situ (n = 1). However, our findings support the previous reports that lesions diagnosed as benign papillomas upon core needle biopsy can be safely managed with clinical and imaging follow-up, and they do not necessarily require surgical excision (8). In our series, one papillary lesion diagnosed as benign upon sonographically guided 14-gauge core needle biopsy was subsequently found to be malignant. Immediate repeat biopsy was recommended in this case due to the imaging-histologic discordance. In a study by Mercado et al. (22), two papillary lesions that were diagnosed as benign upon core needle biopsies were subsequently diagnosed as malignant by surgical excision. Immediate repeat biopsy was recommended in these cases due to the imaging-histologic discordance. Their results and our results confirm the importance of imaging-histologic correlation after core needle biopsy for managing papillary lesions that are diagnosed as benign. In our study, eight (47%) of the 17 atypical biopsies were upgraded to intraductal or invasive carcinoma after surgical excision. Hence, the diagnosis of atypical papilloma or atypical features upon sonographically guided 14-gauge core needle biopsy clearly calls for surgical excision. Underestimation upon core biopsy is an inherent limitation of this technique and this has well been documented (16, 17). This understaging of malignancy upon sonographically guided core biopsy has been shown to decrease with the increasing number of tissue samples, with the use of larger biopsy needles and with the use of a vacuum-assisted biopsy device (14, 15).
Our study has the following limitations. Seven of the 76 papillary lesions were lost to follow-up and so they were excluded from this study. Excluding these cases could have increased the accuracy of 14-gauge sonographically guided core needle biopsy. When diagnosing a lesion as a benign lesion with core needle biopsy, we recommended imaging follow-up and 56% (26/43) of the lesions in our study underwent imaging follow-up with the mean duration of 17.9 months (range: 6-46 months). This was insufficient to accurately estimate the false negative rate.
In conclusion, sonographically guided 14-gauge core needle biopsy of a benign papillary lesion can be reliable when the histological/pathological result is concordant with the imaging characteristics. Surgical excision is indicated for atypical papillary lesions because performing only percutaneous biopsy may underestimate the degree of disease in these cases, yet further study is necessary to confirm our findings.
Figures and Tables
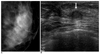 | Fig. 1An asymptomatic 52-year-old woman with benign papilloma upon core needle biopsy.
A. Collimated photograph of the mediolateral oblique mammogram reveals no focal abnormality in the left subareolar area.
B. Sonogram reveals a 7 mm oval mass (arrow) in the left subareolar area. This finding was thought to be concordant with the benign histology of the core biopsy. A subsequent surgical rebiopsy due to the patient's concern also revealed benign papilloma.
|
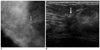 | Fig. 2An asymptomatic 34-year-old woman with benign papilloma upon core needle biopsy.
A. Collimated photograph of the craniocaudal mammogram reveals a 2 cm area of architectural distortion with central density (arrow) in the right subareolar area.
B. Sonogram reveals a 1.6 cm irregular hypoechoic mass (arrow) with a spiculated margin and ductal extension. A subsequent surgical rebiopy was recommended due to the imaging-histologic discordance, and it revealed intraductal papillary carcinoma.
|
Table 1
Comparison of the Sonographically Guided 14-gauge Core Needle Biopsy Results with Surgical Results and Follow-up in 69 Papillary Lesions

Table 2
Comparison of the BI-RADS Categories and the Surgical and Follow-up Results in 69 Papillary Lesions

Note.-*Final assessment categories were also provided according to the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS); category 3 is probably benign lesions, category 4a is low suspicious lesions, category 4b or 4c is moderate suspicious lesions and category 5 is highly suggestive of malignancy.
†One atypical papilloma was stable at 24 months of imaging follow-up.
References
1. Rosen PP. Rosen PP, editor. Benign papillary tumors. Rosen's breast pathology. 1997. Philadelphia: Lippincott-Raven;67–104.
2. Rosen PP. Rosen PP, editor. Papillary carcinoma. Rosen's breast pathology. 1997. Philadelphia: Lippincott-Raven;335–354.
3. Gadd MA. Harris JR, Lippman ME, Morrow M, Hellman S, editors. Papillary lesions. Diseases of the breast. 1996. Philadelphia: Lippincott-Raven;42–45.
4. Haagensen CD. Haagensen CD, editor. Solitary intraductal papilloma. Diseases of the breast. 1986. Philadelphia: Saunders;136–175.
5. Ellis IO, Elston CW, Pinder SE. Elston CW, Ellis IO, editors. Papillary lesions. Systemic pathology: the breast. 1998. 3rd ed. Edinburgh: Churchill Livingstone;133–146.
6. Tavassoli FA. Tavassoli FA, editor. Papillary lesions. Pathology of the breast. 1992. New York: Elsevier, Norwalk; Conn;193–227.
7. Liberman L, Bracero N, Vuolo MA, Dershaw DD, Morris EA, Abramson AF, et al. Percutaneous large-core biopsy of papillary breast lesions. AJR Am J Roentgenol. 1999. 172:331–337.
8. Rosen EL, Bentley RC, Baker JA, Soo MS. Imaging-guided core needle biopsy of papillary lesions of the breast. AJR Am J Roentgenol. 2002. 179:1185–1192.
9. Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998. 208:717–723.
10. Parker SH, Jobe WE, Dennis MA, Stavros AT, Johnson KK, Yakes WF, et al. US-guided automated large-core breast biopsy. Radiology. 1993. 187:507–511.
11. American College of Radiology. Breast imaging reporting and data system (BI-RADSTM) - Mammography. 2003. Reston, Va: American College of Radiology.
12. American College of Radiology. Breast imaging reporting and data system (BI-RADSTM) - Ultrasound. 2003. Reston, Va: American College of Radiology.
13. Gisvold JJ, Goellner JR, Grant CS, Donohue JH, Skyes MW, Karsell PR, et al. Breast biopsy: a comparative study of stereotaxically guided core and excisional techniques. AJR Am J Roentgenol. 1994. 162:815–820.
14. Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, et al. Percutaneous large-core needle biopsy: a multi-institutional study. Radiology. 1994. 193:359–364.
15. Cho N, Moon WK, Cha JH, Kim SM, Kim SJ, Lee SH, et al. Sonographically guided core biopsy of the breast: comparison of 14-gauge automated gun and 11-gauge directional vacuum-assisted biopsy methods. Korean J Radiol. 2005. 6:102–109.
16. Burbank F. Stereotactic breast biopsy of atypical ductal hyperplasia and ductal carcinoma in situ lesions: improved accuracy with directional, vacuum-assisted biopsy. Radiology. 1997. 202:843–847.
17. Jackman RJ, Nowels KW, Rodriguez-Soto J, Marzoni FA Jr, Finkelstein SI, Shepard MJ. Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology. 1999. 210:799–805.
18. Mercado CL, Hamele-Bena D, Singer C, Koenigsberg T, Pile-Spellman H, Higgins H, et al. Papillary lesions of the breast: evaluation with stereotactic directional vacuum-assisted biopsy. Radiology. 2001. 221:650–655.
19. Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dunser M. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999. 173:921–927.
20. Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US-diagnostic yield and tumor characteristics. Radiology. 1998. 207:191–199.
21. Moon WK, Noh DY, Im JG. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology. 2002. 224:569–576.
22. Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006. 238:801–808.
23. Liberman L, Tornos C, Huzjan R, Bartella L, Morris EA, Dershaw DD. Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? AJR Am J Roentgenol. 2006. 186:1328–1334.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download