Abstract
Objective
The presence of an intracerebral hematoma from a ruptured aneurysm is a negative predictive factor and it is associated with high morbidity and mortality rates even though clot evacuation followed by the neck clipping is performed. Endovascular coil embolization is a useful alternative procedure to reduce the surgical morbidity and mortality rates. We report here on our experiences with the alternative option of endovascular coil placement followed by craniotomy for clot evacuation.
Materials and Methods
Among 312 patients who were admitted with intracerebral subarachnoid hemorrhage during the recent three years, 119 cases were treated via the endovascular approach. Nine cases were suspected to show aneurysmal intracerebral hemorrhage (ICH) on CT scan and they underwent emergency cerebral angiograms. We performed immediate coil embolization at the same session of angiographic examination, and this was followed by clot evacuation.
Results
Seven cases showed to have ruptured middle cerebral artery (MCA) aneurysms and two cases had internal carotid artery aneurysms. The clinical status on admission was Hunt-Hess grade (HHG) IV in seven patients and HHG III in two. Surgical evacuation of the clot was done immediately after the endovascular coil placement. The treatment results were a Glasgow Outcome Scale score of good recovery and moderate disability in six patients (66.7%). No mortality was recorded and no procedural morbidity was incurred by both the endovascular and direct craniotomy procedures.
For subarachnoid hemorrhages (SAH) from cerebral aneurysmal ruptures, the incidence of accompanying intracerebral hemorrhage (ICH) is between 4% to 42.5% (7, 10, 13). The accompanying ICH that aggravates the preoperative conditions of patients is widely recognized as a major poor prognostic factor for the treatment of aneurysm. Moreover, the results of previous studies have shown high mortality rates in spite of the active treatments, yet more than 80% of patients died with the conservative procedures only (7, 8, 10); 75-100% died with performing only hematoma removal with no neck clipping of the cerebral aneurysms 1, and 21% to 85% died with performing both neck clipping and hematoma removal (7, 8, 10, 12, 13).
In order to reduce the risk factors of mortality and morbidity that are associated with craniotomy in the surgical high-risk groups of patients, coil embolization of the aneurysm has recently been selectively applied to elderly patients, patients with severe medical problems, patients with posterior circulation aneurysm and patients with a poor preoperative clinical condition (4, 11). The results of this treatment have been reported to be good (2, 3, 6, 11, 14). Therefore, to explore an alternative treatment method that may replace neck clipping and hematoma evacuation in a selected group of patients with aneurysmal ICH, we analyzed and report here on our clinical experiences along with a review of the literature.
From October 2002 to March 2005, we performed coil embolization using the endovascular technique for 119 patients among 312 patients who were admitted with intracerebral subarachnoid hemorrhage. Nine of 65 patients with aneurysmal intracerebral hematoma underwent emergency coil embolizations followed by hematoma evacuation. We retrospectively analyzed their clinical and radiological findings at admission, before and after the embolizations and on follow-up.
The cerebral angiography was done immediately when the brain computed tomography (CT) showed intracranial hypertension with a mass effect in the patients with both SAH and ICH that was caused by an aneurysmal rupture, but the patient's condition was not rapidly worsening. Based on these findings, our team, which included a neurosurgeon and a neuro-interventional radiologist who helped to estimate the treatment options, their risks and their benefits for the patients, decided whether to remove the hematoma after the coil embolization.
Coil embolizations in the cerebral aneurysms were done using digital subtraction angiography by the transfemoral approach. Under general anesthesia, a 6 Fr guiding catheter was first placed as close to the cranial base as possible; a 0.014" microcatheter and a microwire were subsequently put into the cerebral aneurysm via the coaxial technique. With achieving the best angle by viewing the aneurysm neck and parent artery, a Guglielmi Detachable Coil (GDC) (Boston Scientific, Neurovascular Division, Fremont, CA) was inserted using the angiographic road map. A three-dimension or two-diameter coil was preferred for the first coil. Once each coil went in, angiography was used to check its placement, shape and position in relation to the parent vessel before it was released electrically. Inserting the coil was stopped when the contrast agent's inflow was halted or when additional coil insertions were impossible.
Hematoma evacuation was performed as quickly as possible after the coil embolization through the transylvian or transcortical approach. Dissection was minimized for keeping brain damage to a minimum, and the requirement for craniectomy was determined by the severity of the cerebral edema after clot removal. We performed medical management to control the brain edema: injections of hypertonic saline, diuretics and steroid. A review of the patient's prognoses was made using the Glasgow Outcome Scale (GOS) starting at three months after the treatment. We decided that a favorable outcome was the presence of moderate disability or less.
Of the nine total cases, six were male and three female, and their ages ranged from 36 to 62 years (mean: 52.5 years). All of them were grade III or over (two were grade III and seven were grade IV) according to the Hunt-Hess grade (HHG) (Table 1).
The sizes of the intracerebral hematomas (the longest diameters were measured), averaged 4.5 cm and they ranged from 3.5 cm to 7.5 cm. The hematomas were around the sylvian fissure in four cases, mainly in the temporal lobe in three cases and in the frontal lobe in two cases (Table 1).
Cerebral angiograms showed aneurysms in the middle cerebral artery in seven cases, in the internal carotid artery in two cases, on the right side in five cases and on the left side in four. The sizes varied between 2 mm and 17 mm (Table 1).
The follow-up period ranged from three and 20 months. Two patients with HHG III at the time of admission showed good recovery (GR), and of the seven patients with HHG IV, two showed GR, two had moderate disability (MD), and three had severe disability (SD). Overall, six patients showed favorable outcomes, i.e., MD or less (66.7%) and there was no death (Table 2). None of the patients had extraventricular drainage performed for acute hydrocephalus.
A 41-year-old male patient, with as HHG III when admitted, had SAH in the basal cistern and in the right sylvian fissure, and he had ICH in the right frontal lobe (Fig. 1A). As he was suspected of having ICH caused by aneurysmal rupture, an emergency cerebral angiography was performed; a 10 × 11 mm sized aneurysm at the dorsal wall of the right internal carotid artery was found. Coil embolization was subsequently performed (Figs. 1B, C). He was discharged without any major complication; 21 months later, he was graded as GR (Fig. 1D) and is doing well independently.
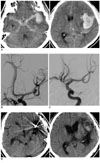
A 56-year-old male with HHG IV was admitted. He had cisternal subarachnoid hemorrhage and ICH at the left temporal lobe (Fig. 2A). Because he was also suspected to have ICH caused by aneurysmal rupture, emergency cerebral angiography was performed; a 3 × 5 mm sized aneurysm at the bifurcation of the middle cerebral artery was found (Fig. 2B). Coil embolization was performed followed by hematoma evacuation (Fig. 2C). No major or minor complications were detected at discharge. Six months later, he was graded as MD (Fig. 2D).
Rupture of cerebral aneurysm mostly causes SAH, but sometimes it causes ICH, intraventricular hemorrhage or subdural hemorrhage that can all occur together (13). The incidence of concomitant ICH caused by an aneurysmal rupture varies from 4% to 42.6% (7, 10, 13). The accompanying ICH causes massive destruction of brain tissue and serious cerebral edema. It not only aggravates the preoperative clinical conditions, but it causes more frequent rebleeding than it does in the patients suffering with only SAH (13). In addition, the condition of ICH patients at arrival to the hospital is usually more critical than that of the SAH-only patients (7-9). We also have found the same findings: all the patients were HHG III or IV on admission.
It is known that the common cerebral arteries whose ruptured aneurysms accompany ICH are mainly the middle cerebral artery bifurcation, the anterior communicating artery, the distal anterior cerebral artery and the internal carotid artery (13). In this study, there were seven middle cerebral artery aneurysms (77.8%) and two internal carotid artery aneurysms (22.2%).
The conventional operative treatment methods for ruptured aneurysms in the patients with ICH include emergency angiography, simultaneous neck clipping and hematoma evacuation. These aggressive treatments are known to improve the conditions of those patients with a low HHG (HHG III, IV and to some extent V) (5). However, the mortality rates are still high, varying from 21% to 85%, whereas the proportion of favorable outcomes is as low as 13% to 48%, which is worse than that for the cases of SAH without ICH (7, 10, 12, 13).
There are several reasons for the failure of the conventional approach to obtain satisfying results. First, there is more severe brain edema in the patients with ICH than in the patients with SAH only. Because of this, an excessive retraction is needed during neck clipping of aneurysm and hematoma evacuation. In addition, a normal retraction can inflict serious damage on the ischemic brain tissue, which in turn will raise the possibility of a secondary cerebral injury as well. This secondary damage will destroy the severely impaired cerebral autoregulation and it aggravates brain edema. Shimoda et al. (12) have reported that 13 out of 27 patients who underwent surgery showed serious brain contusion. Therefore, it is noteworthy that excessive retraction of brain can worsen the patients' conditions even after a successful neck clipping.
Second, a significant arachnoid dissection around the aneurysmal neck is essential as well as controlling the proximal parent artery during the neck clipping. In most cases, the intracerebral hematoma is around the aneurysm; thus, part of the hematoma should be removed to identify its boundary with the neighboring structures, and the neck clipping should be done properly. In this condition, the dissection can cause rebleeding of aneurysm during the hematoma evacuation (9). There have been certain cases where the hematomas were simply evacuated without the neck clipping of aneurysms in order to lower the risk. Yet this is not a desirable procedure because it leaves the aneurysm at the risk for rebleeding after surgery and it does not allow active medical treatment.
Third, a temporary clipping of the parent artery is essential for performing aneurysmal neck clipping. However, it will worsen the ischemic condition of the brain tissue by putting it in infarct, particularly in cases of the aneurysm ruptures that are accompanied by ICH.
Fourth, the difficulty of operative techniques mentioned above is that they prolong the operation time and so increase the chances of complications such as infection, pneumonia, pulmonary edema and heart failure, which will affect the overall prognosis. For these reasons, the conventional operative treatment methods are sometimes not effective in treating the cerebral aneurysm patients with accompanying hematoma.
Various endovascular procedures that are less invasive and more effective are recently being actively employed because they can avoid the risk of direct operations. For all the cases involving endovascular coil embolization, around 9% to 30% are reported to have unwanted results like aneurysm rupture, distal embolism, parent artery occlusion and coil migration; however, these complications can be reduced to 4% to 7% by a well trained performer (6, 14). This has been proved to be a good procedure, and especially for elderly patients or patients with posterior circulation aneurysms, and those patients with a poor preoperative clinical condition (4, 11). In addition, clinical studies have shown that coil embolization has no adverse effects on the patients that are associated with the timing of operation, which proves it to be an effective method to reduce the complications related to vasospasm (2).
However, this technique cannot be applied to all cases with aneurysmal ICH and it needs consideration in several respects. First, the mass effect of a patients' hematoma should not lead to rapid deterioration of the patient's condition. Second, the medical staff as well as the equipment needed to perform emergency cerebral angiography and coil embolization should be in place. Third, the type of aneurysm and its neck size have to be fitted for performing coil embolization because middle cerebral aneurysm, whose rupture occurs most frequently (13), is known to be difficult to apply coil embolization to and such a lesion should be obliterated completely. As long as these conditions are met, this less invasive method will avoid many of the risks of the conventional neck clipping and hematoma removal.
On the reports on conventional operative treatments for the cases with a poor clinical condition on admission, Shimoda et al. (12) have showed that of 47 patients with World Federation of Neurosurgical Society (WFNS) grade IV and V, 38% died and 44% recovered well after neck clipping and hematoma evacuation; in the cases of Tokuda et al. (13) that involved WFNS grades IV and V, these values were was 25% and 21% respectively. Hauerberg et al. (7) has reported that of 91 patients with a poor clinical condition, 43% died and 33% showed satisfactory prognosis. As for coil embolization followed by the hematoma evacuation, Niemann et al. (9) have said in a report on their clinical experience that 24% of 27 patients with WFNS grade IV and V died, while 44% were in good condition. Even though this study included a small number of patients and none with Hunt-Hess grade V, our results are in line with the Niemann's study: there was no death and more than 65% showed to be in a good clinical condition after the procedures.
In this study, we conclude that coil embolization followed by clot evacuation can be an alternative treatment method to neck clipping and hematoma evacuation for the aneurysmal patients suffering with ICH. Further studies are needed to determine these procedures' long term results and their usefulness.
Figures and Tables
Fig. 1
Brain CT shows cisternal subarachnoid hemorrhage and right frontal intracerebral hemorrhage with a mass effect, and an enhanced round mass lesion is seen at the right distal internal carotid artery (A). The emergency right internal carotid artery angiogram demonstrates an aneurysm on the dorsal surface of the internal carotid artery (B). The anterior cerebral artery is shifted, and we occluded the aneurysm with endovascular coil embolization (C). The postoperative three month brain CT shows the coil mass in the aneurysm and small encephalomalatic changes in the right frontal lobe that was previously occupied with a large hematoma (D).
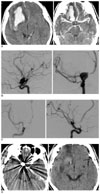
Fig. 2
Brain CT shows all cisternal subarachnoid hemorrhage and left temporal lobe intracerebral hemorrhage with a mass effect by ipsilateral ventricle compression (A). Left cerebral angiogram demonstrates middle cerebral artery bifurcation aneurysm projecting superior-laterally with shifting of M2 portion (B), and occluded sac after coil embolization (C). The postoperative 6 month brain CT illustrates coil inserted state in the sac and encephalomalatic change in the temporal lobe (D).
References
1. Abbed KM, Oglivy CS. Intracerebral hematoma from aneurysm rupture. Neurosurg Focus. 2003. 15:E4.
2. Baltsavias GS, Byrne JV, Halsey J, Coley SC, Sohn MJ, Molyneux AJ. Effects of timing of coil embolization after aneurysmal subarachnoid hemorrhage on procedural morbidity and outcomes. Neurosurgery. 2000. 47:1320–1329.
3. Birchall D, Khangure M, McAuliffe W, Apsimon H, Knuckey N. Endovascular management of acute subarachnoid haemorrhage in the elderly. Br J Neurosurg. 2001. 15:35–38.
4. Byrne JV, Adams CB, Kerr RS. Endosaccular treatment of inoperable intracranial aneurysms with platinum coils. Br J Neurosurg. 1995. 9:585–592.
5. Drake CG. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988. 68:985–986. (Letter).
6. Gonzalez N, Murayama Y, Nien YL, Martin N, Frazee J, Duckwiler G, et al. Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. AJNR Am J Neuroradiol. 2004. 25:577–583.
7. Hauerberg J, Eskesen V, Rosenorn J. The prognostic significance of intracerebral hematoma as shown on CT scanning after aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 1994. 8:333–339.
8. Heiskanen O, Poranen A, Kuurne T, Valltonen S, Kaste M. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien). 1988. 90:81–83.
9. Niemann DB, Wills AD, Maartens NF, Kerr RS, Byrne JV, Molyneux AJ. Treatment of intracranial hematomas caused by aneurysm rupture: coil placement followed by clot evacuation. J Neurosurg. 2003. 99:843–847.
10. Nowak G, Schwachenwald D, Schwachenwald R, Kehler U, Muller H, Arnold H. Intracerebral hematomas caused by aneurysm rupture. Experience with 67 cases. Neurosurg Rev. 1998. 21:5–9.
11. Rowe JG, Molyneux AJ, Byrne JV, Renowden S, Aziz TZ. Endovascular treatment of intracranial aneurysms: a minimally invasive approach with advantages for elderly patients. Age Ageing. 1996. 25:372–376.
12. Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. 1997. 87:170–175.
13. Tokuda Y, Inagawa T, Katoh Y, Kumano K, Ohbayashi N, Yoshioka H. Intracerebral hematoma in patients with ruptured cerebral aneurysms. Surg Neurol. 1995. 43:272–277.
14. Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997. 86:475–482.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download