Abstract
Objective
We wanted to investigate the relationship between the magnetic resonance (MR) findings and the clinical outcome after treatment with non-surgical transforaminal epidural steroid injections (ESI) for lumbar herniated intervertebral disc (HIVD) patients.
Materials and Methods
Transforaminal ESI were performed in 91 patients (50 males and 41 females, age range: 13-78 yrs) because of lumbosacral HIVD from March 2001 to August 2002. Sixty eight patients whose MRIs and clinical follow-ups were available were included in this study. The medical charts were retrospectively reviewed and the patients were divided into two groups; the successful (responders, n = 41) and unsatisfactory (non-responders, n = 27) outcome groups. A successful outcome required a patient satisfaction score greater than two and a pain reduction score greater than 50%. The MR findings were retrospectively analyzed and compared between the two groups with regard to the type (protrusion, extrusion or sequestration), hydration (the T2 signal intensity), location (central, right/left central, subarticular, foraminal or extraforaminal), and size (volume) of the HIVD, the grade of nerve root compression (grade 1 abutment, 2 displacement and 3 entrapment), and an association with spinal stenosis.
Results
There was no significant difference between the responders and non-responders in terms of the type, hydration and size of the HIVD, or an association with spinal stenosis (p> 0.05). However, the location of the HIVD and the grade of nerve root compression were different between the two groups (p< 0.05).
Intervertebral disc herniations are the most common cause of lumbosacral radiculopathy, and 10-15% of patients with these herniations will eventually require surgery (1, 2). Non-surgical care includes bed rest, oral medication, lumbar corsets and physical therapy. Epidural steroid injections (ESI) have historically been used as an adjunct in the treatment of sciatica, and the reported success rates have varied from 20-100%, with an average of 67% (3, 4). This large variation is the result of several methodological and technical differences of this treatment (5). Among the various techniques, recent studies have indicated that fluoroscopic-guided transforaminal ESI with contrast agent injection achieves high success rates in the patients with lumbar disc herniations (6, 7). Nevertheless, the symptoms persist in a significant number of patients and surgery is eventually required.
Many studies have tried to identify the clinical criteria that would predict which patients are most likely to respond to non-surgical treatment (6-10). Others have tried to determine the imaging criteria that would predict which disc herniations are most likely to decrease in size in response to non-surgical treatment (11-16). We undertook a retrospective trial to investigate the relationship between the magnetic resonance (MR) findings and the clinical outcome for lumbar disc herniation patients following transforaminal ESI.
Between March 1, 2001 and August 25, 2002, a total of 263 patients were referred to the Department of Diagnostic Radiology for image-guided percutaneous steroid injections. 91 of 263 patients (50 males and 41 females, age range: 13-78 yrs) were treated with transforaminal ESI for lumbosacral disc herniations. The diagnosis and treatment of the patients was the result of a consensus between an orthopedic surgeon and a musculoskeletal radiologist after having considered the patients' history and the results of physical examinations, plain radiographs, the computed tomography (CT) or MR imaging findings and the electrodiagnostic studies. The ESI indications were 1) a chief complaint primarily of leg pain that did not respond to at least three weeks of conservative treatment with oral medication and physical therapy, except for four patients who needed hospital treatment because of the severity of leg pain, 2) the history, physical examination and pain being consistent with lumbar radiculopathy, and 3) MRI/CT results documenting a herniated lumbar disc with nerve root compression or contact at the level and side of the clinical signs and symptoms. The exclusion criteria were progressive neurological deficits, recent epidural steroid injections, a blood coagulation disorder and an allergic reaction to local anesthetics or corticosteroids. The diagnosis of the patients whose MRI documented small disc herniation with severe central spinal stenosis was regarded as spinal stenosis rather than disc herniation.
Twenty-three of 91 patients whose imaging studies were CT/oust side MRI (n = 13) or whose clinical follow-ups after the injections were missed (n = 10) were excluded in this analysis. A total 68 patients were included in this study with an average follow-up period of 3.6 months (range: 7 days-24 months); 10 patients' follow-up period was within one month, 36 patients' follow-up period was from one to three months, four patients' follow-up period was up to six months and 11 patients' follow-up period was greater than six months. The patients visited our clinic after the injection and they were routinely observed, unless particular symptoms persisted. The outcome was measured using a patient satisfaction scale with choice options of 0 (poor), 1 (fair), 2 (good), 3 (very good) and 4 (excellent), and a visual numeric pain scale ranging from one to 10 at every visit before and after the injection.
The patients were divided into two groups; the successful (responders) and unsatisfactory (non-responders) outcome groups. A successful outcome required a patient satisfaction score greater than two and a pain reduction score greater than 50% at the last visit. 41 of 68 patients (60.3%) had successful outcomes, with an average follow-up period of 4.2 months (range: 3 weeks-24 months). These patients were comprised of 25 men and 16 women with a mean age of 43 years (range: 19-78 years). Twenty-seven patients showed unsatisfactory results, and they had an average follow-up period of 2.7 months (range: 7 days-22 months). These patients were comprised of 12 men and 15 women with a mean age of 41 years (range: 20-67 yrs). 14 of these 27 patients underwent subsequent surgical treatment with an average follow-up period of 1.67 months (range: 7 day-9 months) after the injections.
All procedures were performed in a sterile manner, and fluoroscopic guidance was used for the injections. The patients were placed prone on a fluoroscopy table and the C-arm was rotated to an ipsilateral oblique angle with respect to the suspected nerve root (L4, 5 or S1) (Fig. 1). Lidocaine (1%) was used for the cutaneous and needle tract local anesthesia. After placing a 22 gauge spinal needle at the appropriate level, epidurography with contrast agent was performed to evaluate the anatomy of the epidural space and the distribution of the injection fluid for ensuring that the desired compartment was adequately targeted. We used triamcinolone acetonide suspension 1 cc (TriamcinoloneR 40 mg [Apothecon, Princeton, NJ]) as the long-acting steroid and bupivacain hydrochloride 0.5 cc (MarcaineR 0.5% [Astra USA, Westborough, MA]) as the long-acting local anesthetic. After the procedure, the patient was observed in the short-stay unit for 0.5-1 hour depending on his or her condition. Ten of 68 patients underwent repeat injections; five patients were injected twice, two patients were injected three times, two patients were injected four times and one patient was injected five times. The interval between the injections was three weeks to six months. Although we usually waited 2-3 months before administering repeat injections, the repeat injection was performed in two patients only three weeks after the previous injection. In those patients, we used the half amount of the triamcinolon (20 mg) in the later injections. For the patients who had underwent repeated injections, the requirement of a successful outcome was also a patient satisfaction score greater than two and a pain reduction score greater than 50% at the last visit compared to the score before the injection.
The MR findings were assessed for the type, hydration, location and size (volume) of the herniated disc, the grade of nerve root compression and the associated findings of spinal stenosis. The type of herniated disc was classified as protrusion, extrusion and sequestration. It is important to note that the interpretation of the extruded disc herniation is partly subjective. Hydration of the herniated disc was assessed on the MRI T2 sagittal and axial images, and this was classified as high, moderate or low. The location of the herniated disc was classified as central, left/right central, subarticular or foraminal/extraforaminal according to the recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology (17). The size of the herniated disc was determined by calculating its volume from the axial and sagittal scans. Measurement was performed on a PACS system with drawing the disc and herniation. The grade of nerve root compression was classified as 1 (abutment), 2 (displacement) and 3 (entrapment) (Fig. 2). The findings of associated spinal stenosis (at the central, lateral recess or neural foramen level) were also based on the MR images. Those findings were compared between the responders (n = 41) and the non-responders (n = 27) to the transforaminal ESI.
To determine the relationship between the MR findings and the clinical outcome, the differences between the responder and non-responder groups in terms of the injection level, the type of heriation, the hydration, the location of the herniated disc, the grade of nerve root compression and the association with spinal stenosis were analyzed using Chi-Square or Fisher's exact tests. Student's T test was used for comparing the size of the herniated discs, patient age and gender, and the pre-injection symptom duration between the two groups. A logistic regression analysis was also used to determine the factors contributing to a successful clinical outcome.
The final analysis included 68 patients with an average follow-up period of 3.6 months (range: 7 days-24 months). There were no serious side effects of the injections. Transforaminal ESIs were given at the following levels and all the procedures were done at a single level: L4 in two patients, L5 in 36 patients and S1 in 30 patients.
Forty one of 68 patients (60.3%) had successful outcomes (the responders), and twenty seven patients showed unsatisfactory results (the non-responders). The pre-injection symptom duration of the responders ranged from 3 days to 2.5 years, and that of the non-responders was from 6 days to 2 years. There was no significant difference between the responders and non-responders in terms of injection level, age, gender or the pre-injection symptom duration (p> 0.05). Seven of 41 responders and three of 27 non-responders underwent repeat injections.
On the MR analysis, there was no significant difference between the responders and non-responders in terms of the type, hydration and size of the herniated disc, or an association with spinal stenosis (p> 0.05) (Table 1). However, the location of the herniated disc and the grade of nerve root compression were different between the two groups (p< 0.05) (Tables 2, 3). A centrally located herniated disc was more common in the responder group (Fig. 3), and an extraforaminal disc herniation was also successfully treated (Fig. 4). Treatment of six subarticularly located herniated discs showed unsatisfactory results (Fig. 5). The grade of nerve root compression also correlated with the clinical outcome (Fig. 6). Grade 2 or 3 nerve root compression showed more unsatisfactory results than the grade 1 nerve root compression (odds ratio: 7.43 and 25.9, respectively).
Epidural steroid injection has been used as an adjunct in the treatment of sciatica for a long time. Particularly, the high efficacy of image-guided transforaminal injections were explained by their presumed mechanisms of actions that include precise delivery of the steroid and anesthetic solution (both of which have nociceptive properties), the nerve membrane-stabilizing properties of both steroid and anesthetic, the "wash out" effect of the solution which decreases the levels of the regional inflammatory mediators such as interleukin-1, tumor necrosis factor and phospholipase A2, and the potent anti-inflammatory properties of the steroid (18, 19). Transforaminal ESI also decreases the risk of intravascular injection or dural puncture compared with the traditional techniques that are done without fluoroscopy (6). Nevertheless, a significant number of patients continue to show symptoms after transforaminal ESI, and then surgery is required after several months. Therefore, it would be useful for both patients and clinicians to know the likely outcome of nonsurgical treatments and the likelihood of future surgery.
Several outcome studies of ESI treatment for lumbar herniated discs have evaluated the clinical differences between responders and non-responders (6-10). Lutz et al. have reported that patients with pre-injection symptom durations of less than 36 weeks were most likely to respond to treatment (6). Viton et al. have found that the decrease in pain was greater for the patients less than 50 years of age than for the older patients after transforaminal ESI for lumbar radiculopathy (10). However, the clinical data from these studies were variable and the studies were not in accordance with each other (6-10). The results of the present study indicated there was no significant difference between the responders and non-responders to transforaminal ESI in terms of the injection level, age, gender or pre-injection symptom duration (p> 0.05). In our study, the follow-up period of the non-responders was shorter than that of the responders. That was because most of the non-responders underwent subsequent surgery for pain relief within a short follow-up interval.
Although abnormal imaging findings do not always correlate with the patient's symptoms (20, 21), many studies have investigated the prognostic value of MRI, and they have used MRI to evaluate the morphology changes caused by lumbar disc herniation (11-16). Using MRI, Komori et al. have reported that large sequestered or extruded disc herniations frequently decreased in size after conservative treatment (16). In a study examining the prognostic value of postcontrast MR enhancement in lumbar discs, Gallucci et al. have found that the high signal intensity of the disc fragment on the T2-weighted images were related to a reduction of the disc fragment itself (13). In the present study, there was no significant difference between the responders and non-responders in terms of the type, hydration or size of the herniated disc. The likely explanation for the different result is that the studies of Komori et al. and Gallucci et al. included the patients undergoing only conservative treatment without ESI. Therefore, although their findings indicated that conservative treatment resulted in a size reduction of the large, migrating herniated discs with high signal intensity and the patients showed good clinical outcome, these data could not predict the response of such disc herniations to ESI. Besides, Buttermann reported that ESI does not ultimately alter the regression of the herniated nucleus pulposus, and that patients with less hydrated discs may have more prolonged symptoms, although many improve with ESI (15).
Many studies have reported a poor response to ESI inpatients with disc herniation combined with spinal stenosis or spondylolisthesis (7, 8, 22). Spinal stenosis and spondylolisthesis with a prolonged symptom duration can result in irreversible changes that are related to chronic inflammation, and this may render the nerve root refractory to management by the local application of steroid (7). However, spinal stenosis did not affect the clinical outcome to ESI in the current study because we excluded as such patients from our study.
The present study showed that the location and grade of nerve root compression resulted in significantly different responses to transforaminal ESI. The one case of extraforaminal disc herniation showed a successful result. It was similar with a case reported by Komori et al. (16). In contrast, all the subarticular disc herniations and the disc herniations that severely compressed the nerve root showed unsatisfactory results. It can be assumed that the subarticular location affected the nerve root about the same as a lateral recess stenosis, and severe nerve root compression or entrapment could make the nerve root to be unresponsive to ESI.
It is pertinent to note the limitations of the present study. First, it was not a double-blind controlled study, which may have affected the clinical outcome measurements following transforaminal ESI. Second, the clinical follow-up interval and repeated injection criteria were not uniform since this study was a retrospective trial. Therefore, the evaluation of the transforaminal ESI result according to the time interval was limited. A prospective study with a regular protocol is needed for such an evaluation. Third, the follow-up interval was relatively short, with the average follow-up period being 4.2 months in the responder group. However, the interval between injection and surgery was only 1.67 months (range: 7 days to 9 months) for the non-responders, suggesting that the shortterm outcome of ESI is important for the future management of patients with disc herniation. Finally, although there have been reports on the prognostic value of contrast-enhanced MRI (13, 14) and also reports on the morphological changes observed on follow-up MRI after conservative management of lumbar disc herniation (11, 15), we can not evaluate these since contrast enhancement was not routinely performed, and the follow-up MRIs were obtained in only a few cases.
In conclusion, although the present study has its limitations, the results suggest that MRI could play an important role in predicting the clinical outcome of non-surgical transforaminal ESI treatment for the patients suffering with lumbar disc herniation.
Figures and Tables
Fig. 1
Transforaminal epidural steroid injection.
A. Left anterior oblique radiograph for the right L5 transforaminal injection. The triangle is formed by the iliac crest (IC), the inferior margin of the right transverse process (TP), and the right S1 superior articular process (SAP).
B. Left anterior oblique radiograph for needle positioning. The needle tip is inferior and lateral to the right L5 pedicle (p = pedicle).
C. Epidurography shows the outline of the right L5 nerve root sleeve (arrows).
D. Epidurography for the left L4 transforaminal injection. Contrast is seen outlining the left L4 nerve root sleeve (arrows).
E. Epidurography for the right S1 transforaminal injection (arrows).
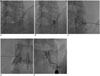
Fig. 2
Grade of nerve root compression.
A. Grade I (abutment); the herniated disc abuts the nerve root, but the nerve root is in its normal position.
B. Grade II (displacement); the herniated disc material displaces the nerve root, but the displaced nerve root can be seen by MRI.
C. Grade III (entrapment); the nerve root is entrapped between the herniated disc and the lamina or facet. The entrapped nerve root is
hardly detected by MRI.

Fig. 3
MRI of a 66-year-old man. Central disc herniation and grade I nerve root compression with a successful result. Mid-line sagittal (A) and axial (B) T2-weighted MR images show central disc protrusion (arrows). The disc was abutting both S1 nerve roots (arrowheads). Because the distribution of the patient's pain was along the right S1 dermatome and the electrodiagnostic study showed right S1 radiculopathy, we performed right S1 transforaminal ESI. The follow-up assessment seven months after injection showed a pain reduction greater than 50% and a patient satisfaction score of 2.
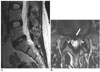
Fig. 4
MRI of a 56-year-old woman. Extraforaminal disc herniation with a successful result. She was suffering from severe posterior thigh and lateral leg pain with tingling sensations. Surgery was planned for this patient as conservative management with oral and intravenous medication had failed.
A-C. The T1-weighted consecutive axial images. Extraforaminal disc herniation (arrow) is observed just above the level of the L5-S1 disc. Note that the disc is located at the extraforaminal course of the left L5 nerve (arrowheads). Left L5 transforaminal epidural steroid injection resulted in remarkable improvement and it removed the need for surgery. Eight months after injection the symptoms had disappeared and she was in a satisfactory condition.

Fig. 5
MRI of a 49-year-old man. Subarticular disc herniation with an unsatisfactory result.
A, B. The T2-weighted consecutive axial images from the L5-S1 disc level. An extruded disc herniation is noted in the subarticular portion of the left L5-S1 (arrows). The nerve root is entrapped in the left lateral recess of S1. Although left S1 transforaminal epidural steroid injection was performed, the symptoms persisted. The patient underwent a disectomy two months after injection.
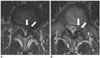
Fig. 6
MRI of a 46-year-old woman. Grade III nerve root compression with an unsatisfactory result. The paramedian sagittal (A) and axial (B) T2-weighted MR images show a left central extruded disc herniation (arrows). The margin of the herniated disc has moderate signal intensity. The left S1 nerve root is not visible due to the herniated disc, as compared to the right S1 nerve root (arrowhead). Left S1 transforaminal epidural steroid injection was performed, but the symptoms persisted. The patient underwent surgery 1.5 months after injection.

References
1. Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica with associated disc pathology: a prospective study with clinical and independent radiologic follow-up. Spine. 1992. 17:1205–1212.
2. Colonna PC, Fredienburg Z. The disc syndrome. J Bone Joint Surg (Am). 1949. 31:614–618.
3. Carette S, Leclaire R, Marcoux S, Morin F, Blaise GA, St-Pierre A, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997. 336:1634–1640.
4. White AH, Derby R, Wynne G. Epidural injections in the diagnosis and treatment of low-back pain. Spine. 1980. 5:78–86.
5. Koes BW, Scholten RJ, Mens JM, Bouter LM. Efficacy of epidural steroid injections for low-back pain and sciatica; a systematic review of randomized clinical trials. Pain. 1995. 63:279–288.
6. Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an out come study. Arch Phys Med Rehabil. 1998. 79:1362–1366.
7. Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002. 27:11–16.
8. Simotas AC. Nonoperative treatment for lumbar spinal stenosis. Clin Orthop Relat Res. 2001. 384:153–161.
9. Papagelopoulos PJ, Petrou HG, Triantafyllidis PG, Vlamis JA, Psomas-Pasalis M, Korres DS, et al. Treatment of lumbosacral radicular pain with epidural steroid injections. Orthopedics. 2001. 24:145–149.
10. Viton JM, Peretti-Viton P, Rubino T, Delarque A, Salamon N. Short-term assessment of periradicular corticosteroid injections in lumbar radiculopathy associated with disc pathology. Neuroradiology. 1998. 40:59–62.
11. Ahn SH, Ahn MW, Byun WM. Effect of the transligamentous extension of lumbar disc herniations on their regression and the clinical outcome of sciatica. Spine. 2000. 25:475–480.
12. Matsubara Y, Kato F, Minatsu K, Nakamura S, Nitta H. Serial changes on MRI in lumbar disc herniations treated conservatively. Neuroradiology. 1995. 37:378–383.
13. Gallucci M, Bozzao A, Orlandi B, Manetta R, Brughitta G, Lupattelli L. Does postcontrast MR enhancement in lumbar disc herniation have prognostic value. J Comput Assist Tomogr. 1995. 19:34–38.
14. Komori H, Okawa A, Haro H, Muneta T, Yamamoto H, Shinomiya K. Contrast-enhanced magnetic resonance imaging in conservative management of lumbar disc herniations. Spine. 1998. 23:67–73.
15. Buttermann GR. Lumbar disc herniation regression after successful epidural steroid injection. J Spinal Disord Tech. 2002. 15:469–476.
16. Komori H, Shinomiya K, Nakai O, Yamaura I, Takeda S, Furuya K. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 1996. 21:225–229.
17. Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology. Recommendations of the combined task forces of the north american spine society, american society of spine radiology, and american society of neuroradiology. Spine. 2001. 26:E93–113.
18. Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine. 1989. 14:431–437.
19. Saal JS, Franson RC, Dobrow R, Saal JA, White AH, Goldthwaite N. High levels of inflammatory phospholipase A2 activity in lumbar spinal disc herniations. Spine. 1990. 15:674–678.
20. Haldeman S. North American Spine Society: failure of the pathology model to predict back pain. Spine. 1990. 15:718–724.
21. Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. a prospective investigation. J Bone Joint Surg Am. 1990. 72:403–408.
22. Rivest C, Katz N, Ferrante FM, Jamison RN. Effects of epidural steroid injection on pain due to lumbar spinal stenosis or herniated disks: a prospective study. Arthritis Care Res. 1998. 11:291–297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download