Abstract
Objective
We wanted to determine whether transcatheter Ethiodol-based capillary embolization in combination with carboplatin could improve the efficiency of a 1:1 Ethiodol-ethanol mixture (EEM) to ablate kidneys that been inoculated with VX-2 carcinoma.
Materials and Methods
The right kidney in 34 New Zealand white rabbits were inoculated with fresh VX-2 tumor fragments. One week later, the kidneys were subjected to transarterial treatment (4-5 rabbits/group): Saline infusion (Group 1); carboplatin infusion (5 or 10 mg, Groups 2A and 2B); carboplatin-Ethiodol (CE) alone (Group 3) and followed by main renal artery occlusion with ethanol (RAO) (Group 4); carboplatin-EEM (C-EEM) followed by RAO (Group 5); carboplatin infusion followed by EEM plus RAO (Group 6); and EEM followed by RAO (Group 7). The animals were followed for up to 3-weeks. The treated kidneys were evaluated angiographically and macroscopically. The kidneys that showed successful embolization macroscopically were entirely cut into serial sections, and these were examined microscopically. Histologically, the kidneys were evaluated on the basis of the residual tumor found in the serial sections.
Results
The results obtained with carboplatin infusion alone (Groups 2A and 2B) and CE without RAO (Group 3) were similar to those of the control animals (Group 1). Kidneys from Groups 4-7 demonstrated macroscopically successful embolization with histologically proven complete renal parenchyma infarction; however, some residual tumor was evident in all but one animal.
Advanced imaging exams are being more frequently performed with newer imaging techniques, and so small renal tumors are being incidentally discovered more often in elderly patients and high-risk patients. This has prompted interest in minimally invasive therapies for the management of small tumors in selected patients. For treatment of kidney tumors, there are reasonably minimal invasive surgical alternatives such as laparoscopic nephrectomy (1) as well as the minimally invasive percutaneous procedures. A variety of energy-based modalities, such as radiofrequency thermal ablation, microwave thermotherapy, laser interstitial therapy, high intensity focused ultrasound and cryoablation have been investigated in that regard (2). Radiofrequency ablation (RF), which is the modality with the most reported experience, has achieved excellent results for treating small exophytic tumors (< 3.5 cm), but successful treatment is less likely as the tumor size increases or the location becomes more central. Complete treatment of most tumors requires one or more overlapping ablations (3). Because the size of the thermal lesion created by RF ablation is influenced by blood flow, which causes heat loss by convection, renal artery embolization was successfully combined by RF ablation to increase the anticancer efficiency (4).
Although transcatheter embolization was the first minimally invasive alternative/adjuvant treatment modality to surgery, its role for the treatment of renal tumors has steadily diminished (5). Yet renal embolization still plays a limited role treating patients who are not surgical candidates, and it is also considered as an adjunctive presurgical therapy; this has recently been shown to provide a survival benefit (6).
Transcatheter embolization is currently used almost exclusively for palliative treatment since it is not considered to be a curative minimally invasive treatment modality. That is mainly because of the imperfect tissue ablation achievable with the embolic materials used in the past. Among the nephron-sparing approaches, transcatheter embolization could, in theory, yield the maximal benefit by offering precise tailoring of the intervention based on the vascular anatomy. Although ethanol alone produces embolization at the capillary level, many studies have shown that some areas of tumor remain viable even in the presence of widespread infarction (7-10). The reason for this non-optimal anti-tumor effect appears to be the inability of the embolic material and administration techniques to achieve complete arteriocapillary infarction.
Ethiodized oils (Ethiodol or Lipiodol, which are iodized ethyl ester of poppy seed oil fatty acids) added to ethanol have proven to be beneficial both experimentally and clinically (11-13). Experimentally, a 1:1 ethiodized-oil/ethanol mixture (EEM) was shown to be an efficacious agent for inducing capillary embolization that was capable of producing complete ablation, provided that concurrent occlusion of the main renal artery was also performed (14). Furthermore, EEM has been proven to be a distinct embolic agent that is distinguishable from ethanol, according to its different mechanism of action (15). A previous rabbit study has shown (16) that 1:1 EEM complemented with pure ethanol injection and/or coil placement had the potential to achieve complete ablation of kidneys that had been inoculated with VX-2 carcinoma, but the treatment was not curative in all cases. Carboplatin is an anti-cancer drug that is available in powder form and so it is suitable to mix with other agents. Carboplatin has been reported to accumulate in VX-2 carcinoma if used in conjunction with temporary flow retardation (17).
The purpose of the study was to establish whether EEM capillary embolization combined with selective intraarterial carboplatin administration could further improve the efficiency of EEM to achieve 100% histologically proven tumor ablation in rabbit kidneys that had been inoculated with VX-2 carcinoma. Also, with using this tumor model, we studied the efficacy of the different components of this combined treatment modality, that is, the effects of short-term intraarterial carboplatin infusion and the effects of ethiodized-oil-enhanced carboplatin chemotherapy with or without arterial occlusion.
All the experimentation involving animals was approved by the Institutional Animal Care and Use Committee at our hospital. The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International) and in accordance with current U.S. Department of Agriculture, Department of Health and Human Services and National Institute of Health regulations and standards.
A total of 34 New Zealand white rabbits (mean weight: 2.57±0.44 kg each, range: 1.5-3.5 kg) was treated and evaluated. Each rabbit was sedated with an intramuscularly injection of 0.5 microgram Buprenex (buprenorphine hydrochloride, Reckitt Benckiser Healthcare [UK] Ltd), and anesthesia was induced and maintained with isoflurane (2-5%) and oxygen (1 L/min). Antibiotics (enrofloxacin, Baytril, 5-mg/kg i.m.; Bayer Corporation, Agriculture Division, Animal Health, Shawnee Mission, KS) were given pre-procedurally and then repeated daily for up to five days. The antero-posterior and lateral baseline chest radiographs were obtained.
The right femoral artery was surgically exposed and a 3-Fr catheter (Cook Inc., Bloomington, IN) was inserted; 100 units/kg of heparin was then administered. Under fluoroscopic monitoring, the catheter was advanced into the right renal artery. Contrast (Conray 60%, iothalamate meglumine, Mallinckrodt Inc., St. Louis, MO) was hand-injected and digital subtraction angiography was used to document the vascular anatomy.
The catheter was then advanced in the mid-portion of the right renal artery beyond the orifice of the capsular artery to avoid tumor seeding in the perirenal tissues. Using a tuberculin syringe, 0.05 mL of freshly harvested and prepared VX-2 tumor fragments (~0.5 mm) were loaded into the catheter, and diluted contrast was used to slowly wash them into the renal vasculature. In an effort to avoid tumor seeding outside of the target vasculature, the catheter was removed without additional injection of contrast material or saline. The right femoral artery was ligated, the wound closed and the animal was allowed to recover from anesthesia. All the inoculations were performed by the same interventional radiologist.
One week after inoculation, the animals were anesthetized as described earlier. The left femoral artery was surgically isolated, a 3-Fr catheter was inserted and 100 units/kg of heparin was administered. Abdominal aortography and selective right renal arteriography were then performed. The kidney was considered suitable for the treatment if no vascular occlusion due to the inoculation was observed more proximal than the interlobular artery level. These arterial occlusions at the interlobular and arcuate levels were considered as indirect evidence for the presence of inoculated tumors. For treatment, the catheter was then advanced into the mid-portion of the right renal artery, beyond the origin of the capsular artery. To avoid inducing spasm in this delicate vasculature, advancement of the catheter was performed without using a guidewire. The positioning of the tip of the catheter into the mid-portion of the renal artery served three goals: 1) it facilitated even distribution of the capillary agent, 2) it helped avoid filling the capsular artery with the capillary agent and/or loosing the carboplatin that had been infused outside the target vasculature, and 3) it utilized the patent capsular artery as a buffering mechanism during injection of the nonradiopaque ethanol to avoid inadvertent embolization.
The inoculated parenchymal VX-2 tumors were divided into 7 groups that were subjected to one of the different locoregional treatments (Table 1, see Methods and Agents).
Group 1, "sham" treatment (control):
10 mL of saline was infused into the main renal artery at a rate of 1 mL/min.
Group 2, intraarterial chemotherapy:
Five mg (Group 2A; n = 2) and 10 mg (Group 2B; n = 4) of carboplatin powder (C) (Paraplatin; Bristol Myers Squibb, Princeton, NJ) mixed with 10 mL of saline was infused at a rate of 1 mL/min.
Group 3, Ethiodol-enhanced chemotherapy:
Carboplatin powder (50 mg) was suspended in 2.5 mL of Ethiodol (Savage Laboratories, Malville, NY) and then it was injected into the main renal artery. No ethanol was injected behind the carboplatin/Ethiodol (CE).
Group 4, Ethiodol-enhanced chemotherapy + arterial occlusion:
Carboplatin powder (50 mg) was suspended in 5 mL of Ethiodol, and a uniform amount (0.30 mL) was injected into the main renal artery. Pure absolute ethanol was then injected in increments (0.1-0.2 mL) until arterial stasis was achieved (CE + ethanol).
Group 5, drug-enhanced capillary embolization + arterial occlusion:
Equal amounts of absolute ethanol (1.5 mL) and Ethiodol (1.5 mL) were drawn into a 3 cc syringe and mixed together by slowly moving an air bubble in the syringe. The 1:1 mixture (EEM) became crystal clear within a minute. A total of 3 mL of the EEM was then injected into the vial containing 50 mg of carboplatin and this was mixed to form a homogeneous emulsion (16.7 mg of carboplatin/mL). The Carboplatin-EEM (C-EEM) was injected slowly and intermittently into the main renal artery until capillary stasis was achieved. Pure absolute ethanol was then injected behind the C-EEM until complete arterial stasis occurred (C-EEM + ethanol).
Group 6, intraarterial chemotherapy + capillary embolization + arterial occlusion:
Ten mg of carboplatin dissolved in 10 mL of saline was infused into the main renal artery at a rate of 1 mL/min. EEM (1:1) was then injected slowly and intermittently until capillary stasis occurred. Pure absolute ethanol was injected in 0.1-mL increments behind the EEM until complete arterial stasis was achieved (C + ethanol).
Group 7, capillary embolization + arterial occlusion:
A 1:1 EEM was prepared as described in Group 5. The EEM was injected into the main renal artery slowly and intermittently until capillary stasis occurred. Pure absolute ethanol was then injected in 0.1-0.2 mL increments until arterial stasis was accomplished (EEM + ethanol).
In summary, carboplatin powder as a chemotherapeutic agent was mixed with Ethiodol or 1:1 EEM and it was also prepared with saline for intraarterial infusion. Ethiodol was used alone as a drug vehicle and also as part of the 1:1 EEM. EEM was used as a capillary embolic agent and also as a carrying agent (drug vehicle). Absolute ethanol as an embolic agent was used exclusively for arterial occlusion following capillary embolization with one of the ethiodized oil based agents.
The agents with Ethiodol were injected into the main renal artery slowly and intermittently until capillary stasis was achieved. Using a tuberculin syringe, the amount of the embolic agent was measured in hundredths of a milliliter. The 0.2 mL of dead-space of the catheter was taken into account during this measurement. Capillary stasis was determined by observing significant slowing of arterial flow and/or by observing the radiopaque oily embolic agent in the renal vein. When it was used, pure absolute ethanol was then injected in increments (0.1-0.2 mL) until arterial stasis was achieved.
After the treatment procedure was completed, the catheter was withdrawn in the abdominal aorta, repositioned above the orifice of the treated renal artery, and then the subtraction aortograms were obtained to establish the post-treatment status (Groups 1, 2) or to document the occlusion of the renal artery (Groups 3-7). The catheter was then removed, the left femoral artery ligated, the incision closed and the animal was allowed to recover from anesthesia. Antibiotics were given daily for five days. All the treatments were performed by the same experienced interventionalist.
Three weeks following treatment, the animal was anesthetized as before. The antero-posterior and lateral chest films, including those of the abdomen, wereobtained. Using either of the femoral approaches, a 3-Fr catheter was inserted into a femoral artery via surgical cutdown, and 100 units/kg of heparin was administered. Abdominal aortograms were obtained to document occlusion or patency of the treated right renal artery. If the artery was patent, then selective renal arteriograms were also obtained. The angiograms were evaluated for arterial patency, parenchymal staining and the presence of tumor.
The animals were sacrificed with using Beuthanasia-D (1 mL; Schering-Plough Animal Health Corp., Kenilworth, NJ), and complete necropsy was then performed. The two largest perpendicular diameters of the kidneys, which were macroscopically much larger than normal, were measured at an accuracy of 5 mm. The measurements involved the perirenal tissues (largely adipose tissues). Because of the presence of significant amounts of perirenal adipose tissue, in the case where the kidney showed a definite reduction in size macroscopically, we did not measure the kidney's diameters. The treated kidneys were fixed in 10% buffered formalin. Those kidneys showing a reduction in size were cut transversally into a series of 2-3-mm thick histological blocks, whereas only a limited number of sections (2-4) were obtained from those kidneys that showed significant enlargement due to uncontrolled tumor growth. Histologic slides were prepared from each section and these were stained with hematoxylin and eosin (H & E) for light microscopic examination. All the histological slides were evaluated by the same board certified veterinary pathologist for the remaining viable kidney parenchyma and tumor. For a semiquantitative analysis, all the slides were scanned (HP scanjet 7400c) into a jpg file with using an overlay grid. For each animal, the total number of squares stained blue/purple per section was counted, as was the total number of squares. The slides were then microscopically assessed to confirm whether the blue/purple squares contained tumor, inflammation or mineral. The number of squares containing tumor was divided by the total number of squares to give a percent of tumor-squares. The amount of remaining viable tumor in each kidney was then established on the basis of the % of the squares that contained viable tumor (Fig. 1).
The histological data concerning the amount of remaining viable tumor in each kidney (% of the grid squares that contained viable tumor) was statistically analyzed. For statistic analysis, the Mann Whitney U-test was performed using the SPSS 11.0 for Windows statistic program (SPSS Inc., Chicago, IL). A p value of less than 0.05 was considered to indicate a statistically significant difference.
As for the tumor growth in the kidney parenchyma, the tumor model based on intraarterial inoculation worked quite dependably. In some cases, the inoculated tumor fragments caused occlusion of vessels at the segmental/subsegmental levels, which rendered these kidneys unsuitable for treatment (the relevant reported numbers do not reflect these kidneys). The peripheral arterial occlusions related to tumor fragments (at the interlobular and arcuate levels) were more frequently observed angiographically. These kidneys were considered as adequate for intraarterial treatment. Leg paralysis related to spinal injury (unrelated to treatment) and surgical cut-down, respectively, resulted in a shorter than expected follow-up period for four animals. The majority (25 of 29) of the treated animals (not including the "sham" treated group) were kept alive until the scheduled sacrifice at 3-weeks after the procedures (Group 2A+B [5/6], Group 3 [3/4], Group 4 [3/4], Group 5 [5/5], Group 6 [4/5], Group 7 [5/5]).
The antero-posterior and lateral chest radiographs were evaluated for pulmonary embolization. None of the radiographs showed any lesions related to venous embolization, even in cases where confluent renal venous filling of Ethiodol-containing embolic agent was observed during and/or after the embolization. In spite of using nonradiopaque pure ethanol for arterial occlusion and withoutemploying a protective balloon catheter, no complications were experienced that could have been caused by inadvertent ethanol injection.
Angiographically, permanent renal artery occlusion was verified in 14 of 19 kidneys (Groups 4-7); angiography was not performed in five kidneys (1 in Group 4 and 4 in Group 6) for technical reasons [such as equipment failure and scheduling conflicts for the animals that were sacrificed earlier than the planned 3-weeks follow-up]. However, all these 19 kidneys (n = 4 in Group 4, n = 5 each in Groups 5-7), were uniformly reduced in size, which indicated renal artery occlusion. These 19 kidneys were cut transversally in their entirety, resulting in a total of 286 slides (mean: 15±2, range: 12-19 slices per kidney) that were examined microscopically. All the other treated kidneys (Groups 2A, 2B, 3) showed completely patent renal arteries angiographically, and no response to the treatment was seen macroscopically. Morphologically, these kidneys were identical to those that received saline infusion ("sham" treatment in the control kidneys Group 1), and there was no difference in the average sizes of the renal specimens. Only a few histological blocks (2-4) that did not cover the total cross sections were randomly selected from these kidneys; these random sections were examined histologically.
Group 1:"Sham" treatment (control) (n = 5)
We started the study with the control group ("sham treatment") to verify the appropriateness of the tumor model. The tumor growth was so aggressive that only one of five rabbits could be kept alive for four weeks after the inoculation (3 weeks after the saline infusion treatment). Two rabbits were sacrificed at 14 days and another two at 18 days after the saline infusion, respectively. Angiograms (Fig. 2) and necropsy verified completely patent renal arteries and enlarged kidneys that were occupied by multifocal tumors (average size: 6.6×4.3 cm). Histologically, the renal parenchyma was replaced by coalescing nodules of VX-2 carcinoma. Some viable renal parenchyma was present and this contained hemorrhage and congestion.
Group 2: Intraarterial chemotherapy (n = 6)
Because we had no previous experience with the dose of carboplatin for renal artery infusion, we initially treated two rabbits with 5 mg of the drug (Group 2A). These two rabbits were followed for 21 and 15 days, respectively. Subsequently, four rabbits were treated with 10 mg of carboplatin (Group 2B) and they were followed for three weeks after treatment. Either dose of the carboplatin had no visible effect on the local growth of the tumor. The average size of the treated kidneys was 7.4×6.1 cm and they were completely occupied by multifocal tumor. Histologically, the renal parenchyma was replaced with coalescing VX2 carcinoma nodules.
Group 3: Ethiodol-enhanced chemotherapy (n = 4)
A mean of 0.46±0.03 mL CE (20 mg carboplatin/mL Ethiodol) was injected over 10±2.8 minutes in four kidneys. Post-procedure aortography verified stasis (there was no parenchymal staining on the subtraction films) with patency of the renal arteries. The average dose of administered carboplatin was 9.15±0.61 mg/kidney. Three renal arteries showed complete patency at the time of the 3-week follow-up; the forth animal was sacrificed at 16 days post-procedure and angiography was not performed due to technical reasons. In all four animals, both macroscopic and microscopic evaluation verified that the tumors were not affected by the treatment. The average size of the kidneys was 6.6×4.5 cm. Histologically, the renal parenchyma was largely replaced by coalescing VX-2 carcinoma.
Group 4: Ethiodol-enhanced chemotherapy + arterial occlusion with ethanol (n = 4)
To decrease the viscosity of the CE emulsion used in Group 3, the amount of carboplatin suspended in the Ethiodol was reduced from 20 mg/mL to 10 mg/mL. A uniform amount of CE (0.3 mL) was injected continuously to achieve capillary stasis. Pure ethanol (0.34±0.15 mL) was injected behind the CE until complete arterial stasis was accomplished. Three animals were sacrificed at three weeks, and one animal was sacrificed at 18 days post-procedure. For technical reasons, no angiography was performed in the latter. Angiographically, all the other three arteries were completely occluded. Grossly, all four kidneys exhibited renal artery occlusion with complete infarction. However, on microscopic examination, variable sized VX-2 tumor nodules were present in the kidneys that were completely infarcted.
Group 5: Drug-enhanced capillary embolization + arterial occlusion with ethanol (n = 5)
Treatment was performed with C-EEM in the five inoculated kidneys in which capillary stasis required the injection of 0.46±0.12 mL of C-EEM during 14.6±7.4 minutes. In three of five kidneys, injection of C-EEM caused arterial stasis and there was no room in the main renal artery to inject ethanol. The calculated mean dose of carboplatin was 7.7±1.9 mg/kidney (range: 5.8-10.5 mg). At three weeks post-procedure, all the renal arteries remained completely occluded; macroscopically, all the kidneys were significantly reduced in size (Fig. 3).
Histologically, the renal parenchyma in all the kidneys was completely infarcted. The capsular surface and the renal hilus contained granulation tissue and inflammation, which was indicative of tissue repair/resorption. Some tumor growth was evident in all the animals, but the amount of tumor growth was significantly less than that seen in Groups 1-3.
Group 6: Intraarterial chemotherapy + capillary embolization + arterial occlusion with ethanol (n = 5)
Five inoculated kidneys were treated with a short-term infusion of 10 mg of carboplatin, and this was followed by capillary embolization with 1:1 EEM. Achieving capillary stasis required 0.45±0.09 mL of EEM that was injected during 9.2±2.4 minutes. Pure ethanol (0.25±0.2 mL) was injected behind the EEM, resulting in complete arterial stasis in four kidneys. One animal had to be sacrificed at two days post-procedure because of leg paralysis related to the surgical cut-down. The other four animals were followed for three weeks. For technical reasons, no angiography was performed in three of these animals. Necropsy revealed that all the kidneys harvested at three weeks were reduced in size, which indicated macroscopically successful embolization.
Histology revealed complete parenchyma infarction in all the kidneys, with some residual tumor growth in all but one kidney. The one kidney that was harvested two days after the treatment was completely tumor-free.
Group 7: Capillary embolization + arterial occlusion with ethanol (n = 5)
A mean of 0.38±0.07 mL of 1:1 EEM that was injected during 32.8±5.5 minutes led to capillary stasis. Complete arterial stasis was achieved in all kidneys ; in four kidneys, this required injection of pure ethanol (0.15 mL for each of 4 kidneys). Angiographically, all the renal arteries remained completely occluded at 3-weeks (Fig. 4). Necropsy revealed that all the kidneys were significantly reduced in size. Histologically, complete parenchymal infarction was found with some tumor growth in all kidneys.
The histological data concerning the amount of remaining viable tumor in each treated kidney (% of the grid squares containing viable tumor) was statistically analyzed and compared (Table 1). Comparing Groups 2 and 3 to Group 1 (control), the amount of viable remaining tumor was significantly less in Group 2, but not in Group 3. Compared to the control group (75.5±14.5), significantly less viable tumor was found in the treated kidneys of the animals in Groups 4-7 (25.7±6.5, 14.2±10.6, 15.2±13.3, 23.0±16.0; p = 0.021, 0.014, 0.014, 0.014, respectively), indicating there was efficient treatment by the different ethiodized oil based modalities and combined with successful arterial occlusion (Fig. 5). However, there was no statistical difference among groups 4, 5, 6 and 7, and this implied that none of the treatments increased the efficacy of the 1:1 EEM embolization (Groups 4-6 vs. Group 7). The tumors found in the completely infarcted renal parenchyma may have resulted from residual tumors, local recurrences from perirenal components of the original lesions or from metastases that occurred during the 3-week follow-up (Fig. 6).
Previous studies have shown that addition of ethiodized oil to ethanol is beneficial and it helps facilitate homogeneous distribution (12) and retention of alcohol in the capillaries (11). Based on these observations, other studies have been conducted to further investigate the possibilities of using 1:1 EEM renal embolization (14-16). It was nitially determined that EEM injections alone did not produce permanent capillary embolization in normal porcine kidneys. Only prompt and permanent occlusion of the main renal artery could preserve the capillary embolization achieved with using EEM. Based on these findings, a two-phase embolization technique consisting of homogeneous capillary embolization with 1:1 EEM and ethanol/mechanical occlusion of the main arterial compartment facilitated by temporary balloon occlusion, was developed for achieving histologically complete renal ablation in normal swine (14). In a rabbit study, the temporal histopathological changes after renal embolization with 1:1 EEM were evaluated to elucidate the mechanism of action (15). The results showed that 1:1 EEM did not produce immediate spasm, arterial wall damage or early parenchymal changes, which allowed the EEM to penetrate all the way through the capillaries (15). These parenchymal events are distinctively different from those produced by ethanol alone when following intraarterial injection, that is, the early fibrinoid necrosis of the glomerular tufts and the glomerular changes are consistent with direct cellular toxicity rather than thrombosis-induced hypoxia (18). EEM capillary embolization combined with main renal artery occlusion via ethanol injection prevents the EEM from passing through the capillaries to the venous side. Based on the different mechanism of actions, 1:1 EEM is considered to be a distinct embolic agent that differs from pure absolute ethanol (15).
In a rabbit study, 1:1 EEM was administered in combination with pure ethanol and coil placement for achieving histologically complete transcatheter ablation of kidneys that were implanted with VX-2 carcinoma (16). Three weeks after the procedure, the treated kidneys were sectioned and evaluated histologically for viable tumor. Altogether, five of 12 kidneys (41.6%) and 139 of 151 slides (92.1%) proved to be tumor free. However, some microscopic viable tumors were found, indicating that renal embolization with using 1:1 EEM as a capillary agent in combination with other embolic agents (ethanol, coil) effectively controlled the VX-2 tumor that was confined to the kidney parenchyma in some of the rabbits, but it was not curative in the majority of cases. It was speculated that one possible reason for the presence of residual tumors was the failure to fill the entire renal vasculature with EEM.
Carboplatin, an anticancer agent, was selected for our study because it is available in powder form, which allows it to mix with Ethiodol. In an acute study, the correct carboplatin concentration, following systemic, intraarterial and locoregional administration of absorbable gelatin powder (Gelfoam) and starch microspheres (Spherex), was assessed for treating VX-2 tumors that were implanted into rabbit liver (17). However, we thought the use of 50 mg of carboplatin per animal was too high for a chronic study. Our bench-top experiments showed that no more than 10-20 mg of carboplatin powder could be added to one mL of Ethiodol or EEM for locoregional application, and that locoregional administration of the carboplatin with using the given drug vehicles (Ethiodol, EEM) would technically allow for delivery of not more than 10 mg of carboplatin per kidney. The dose for intraarterial infusion was determined accordingly. In that way, the efficacy of the different treatments remained comparable as far as the carboplatin dose-range was concerned.
As for the efficacy of the carboplatin, when used in different forms for treating inoculated VX-2 tumors, the study has shown that adding carboplatin to the two-stage ethiodized oil-based capillary embolization did not improve the overall effectiveness. When the carboplatin was used alone, its short-term intraarterial infusion (either the 5 mg or the 10 mg) was not effective to achieve local tumor control and its efficacy did not differ from that of the saline infusion. When administered as carboplatin/Ethiodol (CE) without arterial occlusion, this mixture produced capillary stasis in all cases at the end of injection, but the effect proved only temporary. The renal arteries were found to be completely patent at 3-weeks post procedure and the tumors were not affected by the treatment. Without prompt and permanent renal artery occlusion, the CE washed out of the arteriocapillary bed, allowing the tumors to continue growing out of control. Because this phenomenon was observed in our previous studies (14), the findings from this group prompted us to repeat the CE treatment with arterial occlusion in four kidneys (Group 4). At the 3-week follow-up, all 4 kidneys showed homogeneously increased density (mineralization plus retained ethiodized oil) on plain films, along with a significant reduction in size. Histologically, no viable kidney parenchyma was present, but the examined kidneys showed an average of 29% residual tumor. Nevertheless, permanent arterial occlusion resulted in macroscopically successful embolization with only a 3 mg dose of carboplatin per kidney. The fact that these kidneys did not differ from the kidneys in Groups 5-7 may indicate the negligible role of carboplatin in the result of the Group 4 kidneys.
Furthermore, there was no difference among the other responder groups either (Group 5-7). In comparison to Group 7 (capillary embolization + arterial occlusion), neither the capillary embolization performed with carboplatin-EEM supplemented with arterial occlusion (Group 5) nor the short-term intraarterial carboplatin injection, which was used prior to EEM embolization, and followed by arterial occlusion (Group 6) resulted in a better overall outcome. Again, the addition of carboplatin to the treatment regimen in two different forms did not improve the results obtained from the original two-stage capillary embolization.
Histological analysis (286 histological slides/19 kidneys) demonstrated macroscopically successful embolization with histologically proven complete renal parenchymal infarction in Groups 4-7. In contrast, viable tumor growth was evident in all the animals except one from Group 6, which was euthanized two days after treatment. However, this tumor growth was significantly less than that observed in the control animals (Group 1). The kidneys that responded to treatment in Groups 4-7 were macroscopically identical. The histology did not reveal any statistically significant difference among these kidneys, based on the residual viable tumor. These observations indicate that the addition of carboplatin in any form did not increase the therapeutic efficacy of 1:1 EEM embolization, nor did it render the kidneys tumor-free. Simultaneously, these observations also suggest that the complete renal parenchymal infarction seen in Groups 4-7 was achieved by using some form of ethiodized-oil based capillary embolization, followed by prompt and permanent renal artery occlusion with ethanol. In that regard, all these favorable results may be uniformly attributed to ethiodized oil capillary embolization complemented by renal artery occlusion, and this is irrespective of the use of carboplatin at all; the key factor here is the use of the ethiodized oil as an efficacious capillary agent. Since the completion of the reported study, this assumption has already been confirmed by a pilot study in which pure ethiodized oil was used alone as a single capillary agent, and this was followed by arterial occlusion (19).
One of the main limitations of the study is that it used only a single time-point for evaluation. Consequently, the origin of the viable tumors observed 3-weeks after treatment could not be determined. The presence of viable tumors in the shrunken kidneys 3-weeks after treatment may be attributed to real "residual tumors" that have survived the ablation. Nevertheless, the tumors grew much more rapidly in the non-responder kidneys and by the end of 3-weeks they had formed large coalescent tumor noduli in the considerably enlarged kidneys. The observed tumors may also be attributed to activation of dormant tumor cells by growth factors and angiogenesis, but it is also possible that extrarenal tumor cells invaded the granulation tissue and partially replaced the completely infarcted renal parenchyma. These observations underline the necessity of using multiple time-points for adequately evaluating the efficacy of the various treatments.
The treatment protocol was a bit uneven, and this was caused by some technical problems. In some cases, angiography was not performed at the time of tissue harvest (Groups 4 and 6). Yet the signs on the plain radiographs and the macro- and microscopic evaluations were identical to those observed after angiographically proven renal artery occlusions. In Group 5, ethanol was not injected into the renal artery in 3 of 5 kidneys because the C-EEM had already occluded the arterial compartment. Follow-up angiography verified complete arterial occlusion of these renal arteries, so the lack of ethanol injection was not considered as a diversion from our protocol, which aimed at establishing prompt and permanent arterial occlusion so as to preserve the effect of capillary embolization.
As for possible clinical use, a method of transcatheter embolization that could achieve total tissue/tumor ablation would be a curative alternative to minimally invasive surgical procedures, as well as to percutaneous energy-based minimally invasive treatments. The operative times, blood loss, procedure-related morbidity, the degree of invasiveness, patient discomfort and pain, and the length of the hospital stay are all factors that favor catheter-based interventions. Also, the capillary embolization technique could be utilized for tumors without size limitation (20).
More research is needed to develop an "ideal" embolic agent that's capable of ablating tissues with the efficiency of surgical removal. As a first step, the part of this study that poduced macroscopically successful results with histologically verified residual tumors, in spite of complete parenchymal infarction, should be repeated with an adequately shortened follow-up time points. Further studies are warranted to elucidate the mechanism of action of ethiodized oil-based capillary embolization that's combined with prompt and permanent arterial occlusion.
Figures and Tables
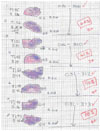 | Fig. 1The amount of residual tumor remaining in the kidney at post treatment was assessed from H & E stained sections of paraffin-embedded, formalin-fixed tissues (see text). The figure contains all the slides (2/kidney) analyzed for the non-responder kidneys from Group 1 ("sham" treatment). |
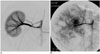 | Fig. 2"Sham treatment" (03L-306).
A. Selective right renal arteriogram (for technical reasons, the access was created from the right carotid artery): Normal right kidney (electronic magnification 1X).
B. Selective right renal arteriography: 25-day post inoculation (18-day post saline infusion): The right kidney is enormously increased in size and is completely occupied by multifocal tumors (same electronic magnification)
|
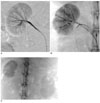 | Fig. 3Carboplatin-EEM Embolization (04L-408).
A. Right renal arteriogram: Normal vasculature (electronic magnification 2X).
B. Plain film obtained immediately after embolization. 0.42 mL of embolic agent completely filled the arteriocapillary system: The parenchyma stains densely and homogeneously, while the segmental-interlobar-interlobular arteries are almost contiguously filled. The EEM embolization resulted in complete arterial stasis; no ethanol was injected into the renal artery. Note that the capsular artery is also occluded.
C. Plain abdominal film obtained 3-week post treatment. The right kidney is reduced in size, and its parenchymal density is almost homogeneously increased. The left kidney shows compensatory hypertrophy.
|
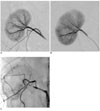 | Fig. 4EEM embolization (03L-326).
A. Normal selective right renal arteriogram (magnification 1X).
B. Selective right renal angiogram 1-week post VX-2 tumor inoculation: Several peripheral branches are occluded at the interlobar-interlobular-arcuate level throughout the kidney, the intensity of the parenchymal staining is slightly uneven (magnification 1X).
C. Selective right renal arteriogram obtained 3-weeks post EEM embolization: The right renal artery is occluded, except for a 1-cm long stump that carries the patent capsular artery. The capsular artery increased in caliber, (its branches supply the perirenal soft tissue), but there is no parenchymal staining. Note that the right kidney is slightly reduced in size with moderately increased radiopacity, indicating residual Ethiodol and mineralization (normal view).
|
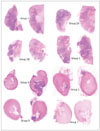 | Fig. 5A comparison of tumor retention and renal embolization between the control and treated groups. Kidney sections from two animals from each group are scanned using the HP scanjet 7400c (magnified x 2). Group 1 is the control and it has the most tumor, as demonstrated by the blue/purple areas (see also Fig. 1) and this was confirmed histologically as being VX2 carcinoma. The tumor is diffusely spread throughout the kidney. Groups 2A, 2B and Group 3 have less VX2 carcinoma than the control group, and the tumor is still distributed throughout the renal parenchyma. Groups 4, 5, 6 and 7 are all similar to each other and they are very different from Groups 1, 2A, 2B and 3. However, Group 4 appears to have more blue/purple tumor than Groups 5, 6 and 7. The kidneys of these latter 4 groups are smaller and completely embolized. The remaining tumor is not diffusely distributed throughout the parenchyma, but it is generally at the capsular surface, in the attached peri-renal fat or in the pelvis of the kidney. The blue/purple is either tumor or inflammation or mineralization, and it frequently shows all three in the sections (see Fig. 6). |
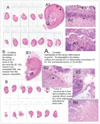 | Fig. 6Embolized kidneys from Groups 6 and 7 demonstrating the pattern of tumor re-growth.
A. The entire kidney is sectioned, demonstrating a smaller kidney that is completely embolized. No tumor was present in any section (score = 0) on microscopic examination. A2 (magnified x 25) and A3 (magnified x 400) demonstrate that at the capsular surface, the outermost blue/purple is mineralization (arrow), and the inner blue/purple (arrowhead) at the junction with the necrotic parenchyma is inflammation.
B. The entire kidney is sectioned and this includes some adherent peri-renal fat containing blue/purple nodules (*) that are VX2 tumors. In B2 (magnified x 25), the tumor (*) is in the peri-renal fat, and the fat is separated from the renal parenchyma by blood vessels (bv) in the granulating renal capsule. The blue/purple (arrow) below the capsule is mineral on microscopy. B3 (magnified x 25) is a tumor (*) in the medulla of the same section that appears to arrive from a tumor's embolized vessel (bv). B4 (magnified x 100) demonstrated that the tumor (*) invades from the capsular surface into the pink embolized renal parenchyma.
|
References
1. Cobb WS, Heniford BT, Matthews BD, Carbonell AM, Kercher KW. Advanced age is not a prohibitive factor in laparoscopic nephrectomy for renal pathology. Am J Surg. 2004. 70:537–542.
2. Murphy DP, Gill IS. Energy-based renal tumor ablation: a review. Semin Urol Oncol. 2001. 19:133–140.
3. Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of renal cell carcinoma. Eur Radiol. 2005. 15:960–967.
4. Yamakado K, Nakatsuka A, Kobayashi S, Akeboshi M, Takaki H, Kariya Z. Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience. Cardiovasc Intervent Radiol. 2006. 29:389–394.
5. Lanigan D, Jurriaans E, Hammonds JC, Wells IP, Choa RG. The current status of embolization in renal cell carcinoma - a survey of local and national practice. Clin Radiol. 1992. 46:176–178.
6. Zielinski H, Szmigielski S, Petrovich Z. Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol. 2000. 23:6–12.
7. Craven WM, Redmond PL, Kumpe DA, Durham JD, Wettlaufer JN. Planned delayed nephrectomy after ethanol embolization of renal carcinoma. J Urol. 1991. 146:704–708.
8. Klimberg I, Hunter P, Hawkins IF, Drylie DM, Wajsman Z. Preoperative angioinfarction of localized renal cell carcinoma using absolute ethanol. J Urol. 1985. 133:21–24.
9. Imai S, Kajihara Y, Nishishita S, Hayashi T. Effect of ethanol induced occlusion of the renal artery in rabbit kidney implanted with VX2 carcinoma. Acta Radiol. 1989. 30:535–539.
10. Ellman BA, Parkhill BJ, Curry TS 3rd, Marcus PB, Peters PC. Ablation of renal tumors with absolute ethanol: a new technique. Radiology. 1981. 141:619–626.
11. Park JH, Jeon SC, Kang HS, Im JG, Han MC, Kim CW. Transcatheter renal arterial embolization with the mixture of ethanol and iodized oil (Lipiodol®). Invest Radiol. 1986. 21:577–580.
12. Wright KC, Loh G, Wallace S, Stephens LC. Experimental evaluation of ethanol-ethiodol for transcatheter renal embolization. Cardiovasc Intervent Radiol. 1990. 13:309–313.
13. Park JH, Han JK, Chung JW, Choi BI, Han MC, Kim YI. Superselective transcatheter arterial embolization with ethanol and iodized oil for hepatocellular carcinoma. J Vasc Interv Radiol. 1993. 4:333–339.
14. Konya A, Wright KC. Capillary embolization using ethiodol-ethanol for complete renal ablation in Swine. Invest Radiol. 2002. 37:512–520.
15. Konya A, Van Pelt CS, Wright KC. Ethiodized oil-ethanol capillary embolization in rabbit kidneys: temporal histopathological findings. Radiology. 2004. 232:147–153.
16. Kónya A, Wright KC. Ethiodol-ethanol capillary embolization in rabbit kidney implanted with VX-2 carcinoma. Radiological Society of North America (RSNA) Scientific Assembly and Annual Meeting Program 2001. Radiology. 2001. 221(P):184.
17. Pohlen U, Berger G, Binnenhei M, Reszka R, Buhr HJ. Increased carboplatin concentration in liver tumors through temporary flow retardation with starch microspheres (Spherex) and gelatin powder (Gelfoam): an experimental study in liver tumor-bearing rabbits. J Surg Res. 2000. 92:165–170.
18. Buchta K, Sands J, Rosenkrantz H, Roche WD. Early mechanism of action of arterially infused alcohol U.S.P. in renal devitalization. Radiology. 1982. 145:45–48.
19. Konya A, Stephens CL, Wright KC. A new transcatheter method for renal ablation: pure ethiodized oil is an efficient ablative agent. 2005. In : Radiological Society of North America (RSNA) Scientific Assembly and Annual Meeting Program; 409.
20. Kauffmann GW, Richter GM, Rohrbach R, Wenz W. Prolonged survival following palliative renal tumor embolization by capillary occlusion. Cardiovasc Intervent Radiol. 1989. 12:22–28.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download