Abstract
Objective
We wanted to evaluate the effectiveness of intraluminal irradiation with Holmium-166 (166Ho) for reducing the pseudointimal hyperplasia (PIH) in the transjugular intrahepatic portosystemic shunt (TIPS) tract in a swine model.
Materials and Methods
TIPS was performed in 12 domestic pigs, after the creation of portal hypertension by intraportal injection of a mixture of N-butyl-2-cyanoacrylate (NBCA) and lipiodol. Five pigs first underwent intraluminal irradiation (30 Gy) in the parenchymal tract with using a 166Ho solution-filled balloon catheter, and this was followed by the placement of a nitinol stent in the TIPS tract. For the seven control pigs, the balloon was filled with saline and contrast media mixture. Two weeks later, follow-up portography and histological analysis were performed.
Results
TIPS was successfully performed in all twelve pigs with achieving artificially induced portal hypertension. Portography performed two weeks after TIPS showed the patent tracts in the TIPS tracts that were irradiated with 166Ho (5/5, 100%), whereas either completely (5/6, 83.3%) or partially (1/6, 16.7%) occluded TIPS were seen in the seven pigs of the nonirradiated control group, except in one pig that experienced periprocedural death due to bleeding. Histological analysis showed a statistically significant difference for the maximal PIH (irradiated: 32.8%, nonirradiated: 76.0%, p < 0.001) between the two groups.
The transjugular intrahepatic portosystemic shunt (TIPS) procedure is widely used for portal decompression in the setting of portal hypertension complicated by variceal hemorrhage or intractable ascites. However, the follow-up data has demonstrated the limited long-term patency of TIPS, and the resultant recurrent portal hypertension and rebleeding have occurred by one year in up to 50-60% of the cases due to stenosis or occlusion of TIPS (1).
The previous clinical and experimental studies have reported that the causes of TIPS malfunction were thrombosis during the early stage (within 2 weeks), and pseudointimal hyperplasia (PIH) within the stented lumen of the hepatic parenchymal tract and intimal hyperplasia within the outflow hepatic vein during the delayed stage (2-4). Histologically, PIH, which is the main cause of stenosis, is initially made of loose granulation tissue that's formed by edema, myofibroblasts, neo-capillaries, collagen fibers and inflammatory cells. Thereafter, it is thickened by the increased myofibroblasts and collagen fibers. PIH in TIPS has known to be pathogenically similar to myointimal hyperplasia after arterial stenting, and the over-proliferation that occurs in both processes leads to luminal stenosis and stent failure (3). The pathophysiology of PIH remains unclear, but biliary leakage into the shunt, exposed liver parenchyma and injured hepatic veins have been suggested as being the initiators of hyperplasia (1, 4). However, their significance is not unanimously accepted (5).
Irradiation with using external X-ray beam or intraluminal brachytherapy was originally introduced for treating the stent stenosis, following arterial myointimal hyperplasia, that occurred after coronary or noncoronary arterial intervention, and this type of irradiation treatment has been successfully performed and reported upon (6-10). Based on the similar proliferating processes of myointimal hyperplasia and PIH, irradiation therapy was thought to be useful for limiting the PIH in TIPS. However, its utility for treating PIH in TIPS has not been validated. To the best of our knowledge, there has been only one experimental study by Lessie et al. that has evaluated the ability of intraluminal irradiation to treat PIH in TIPS (11). They applied irradiation for TIPS in a swine model and they reported that a low-dose (15.2 Gy) of beta irradiation, which has been commonly used for arterial stent treatment, did not significantly reduce the development of PIH in TIPS. They explained that this negative result could be caused by the differences in the origin of the proliferating cells and their migration distance between myointimal hyperplasia and PIH, and an insufficient irradiation dosage was also a factor.
We hypothesized that administering a higher dose of beta irradiation than the dose used in the coronary intervention to the hepatic parenchymal tract at the time of TIPS creation could inhibit hyperplasia and preserve stent patency. Therefore, the purpose of this study was to evaluate the ability of intraluminal irradiation with high-dose (30 Gy) Holmium-166 (166Ho) to reduce hyperplasia and prevent stenosis of TIPS in a portal hypertension-induced swine model.
The experimental protocol of our study was used in all 12 domestic pigs (Yorkshire pigs, 2 months old and weighing 15-20 kg each), after approval by the animal care committees at this institution. All the pigs were in good physical condition prior to the experiment. The techniques and instrumentations used to create TIPS were similar to that used for humans. This investigation aimed to compare the results of the irradiated stent with the control stent at a follow-up interval of two weeks. Five pigs of the irradiated group underwent intraluminal irradiation of the intrahepatic parenchymal tract during balloon inflation of TIPS, whereas the seven pigs in the control group underwent TIPS without irradiation.
166Ho is a beta-emitting radioisotope. In our study, the targeted therapeutic dose for TIPS was set at 30 Gy. Prior to the experimental study in animals, we conducted an in vitro pilot study to determine the optimal concentration activity and exposure time of 166Ho for acquisition of the desired absorbed radiation dosage.
The liquid form of 166Ho-(NO3)3 5H2O-DTPA was provided by the Korea Atomic Energy Research Institute (KAERI, Taejon, Korea). It was prepared by a neutron (n, γ) reaction on abundant, non-radioactive 165Ho that was at a thermal flux state and by simple mixing the 166Ho with DTPA at room temperature. The produced 166Ho had a specific activity of 7.4 GBq mg·-1 with a physical half-life of 26.8 hour, and it emitted high energy β-particles with maximum energies of 1.85 MeV (51%) and 1.77 MeV (48%). A portion of γ rays and K-shell X-rays were also emitted. The range of absorbed β-particles distribution was 8.6 mm at maximum and 1.2 mm on average (12, 13).
GafChromic film (MD-55, Model Mo. 37-041, Lot #941206, ISP Technologies Inc., Wayne, NJ) was used for estimating the radiation dosage. A 60Co teletherapy beam and a 6 MV photon beam were used to obtain the dose optical density calibration curves for the GafChromic film. The exposed films were read by using a videodensitometer (Model # WP700, Weellhofer Co., Schwarzenbruck, Germany), with changing the radiation dosage. The angioplasty balloon catheter (10 mm-diameter ×4 cm-length, Cook, USA) was filled with 3.16 mL of 166Ho solution (concentration activities: 1,850 MBq (50 mCi/mL) and 3,700 MBq (100 mCi/mL)) and the balloon was inflated to 3 atm pressure. The balloon was positioned in a solid water phantom, and GafChromic film was placed and adhered closely on the surface of balloon at an orthogonal direction.
The exposure times for the desired absorbed dose (30 Gy) at the balloon surface were calculated with using the different concentration activities (1,850 MBq and 3,700 MBq) (Table 1). Finally, the balloon was filled with 166Ho at a concentration activity of 1,850 MBq and it was inflated to 3 atm for 4.5 minutes, delivering an absorbed surface dosage of 30 Gy, which was the dose we decided to use in the experimental study.
The animals were initially sedated with intramuscular injections of 2 mL ketamine hydrochloride (Ketalar, Yuhan, Seoul, Korea) and 2 mL xylazine hydrochloride (Rompun, Hayer, Seoul, Korea) and sedation was maintained by an intravenous administration of ketamine hydrochloride (0.01 cc/mL, 1 gtt/sec) during the procedure. The pigs were placed in the supine position for flouoroscopy (Integris 2000, Philips, the Netherlands) with their necks were rotated to the left. After making a skin incision (1-2 cm) under sterile conditions, right external jugular venous access was achieved by using an 18-gauge micropuncture introducer (Green I.V. catheter®, Greencross Medical Co., Seoul, Korea), a 0.035-inch Terumo guide wire (Terumo Corporation, Tokyo, Japan) and a coaxial catheter-needle set (RUPS 100, Cook, Charenton, France). A 10-Fr sheath without an inner needle (Cook) was passed to the inferior vena cava and advanced caudally into the right (or middle) hepatic vein. After catheterization of the right hepatic vein, a hepatic venogram and wedge portogram were performed to guide the puncture of the right portal vein. A 16-gauge Colapinto needle set (Cook) was introduced into the hepatic vein. It was rotated ventrally and the tip was firmly fixed against the vein wall. The needle and a 5-Fr catheter were passed into the parenchyma toward the anticipated region of the portal vein. After puncture of the right portal vein, this was confirmed by aspiration of venous blood and by injecting a small amount of contrast agent; the needle was removed, and negative pressure was applied to the 5-Fr catheter during slow withdrawal. Once the portal vein was punctured, a 0.035-inch Terumo guide wire and a 5-Fr angiographic catheter were advanced into the main portal vein, and the pressure gradient between the portal vein and the vena cava was measured using a Pharmaseal central venous pressure monitor (Baxter Healthcare Corporation, Valencia, CA). Portal venography was also obtained with injecting contrast media by hand, and this demonstrated the portal venous anatomy and its diameter.
Portal hypertension was artificially induced by intraportal injection of 0.02 mL of N-butyl-2-cyanoacrylate (NBCA, sclerosing embolic agent, B-Brawn, Cook) mixed with lipiodol (Lipiodol® ultra-fluid, Guerbet, France) (1:3). Under fluoroscopy, this mixture was injected until stasis of the portal flow was achieved, and the portal pressure was measured to confirm the occlusion. The injection volume of the embolic mixture was approximately 1.5-2 mL. After removal of all the needle parts except the superstiff Amplatz-type guide wire and outer sheath, an angioplasty balloon catheter (10 mm in diameter, 4 cm in length) was used to dilate the hepatic parenchymal tract. Balloon dilatation was performed at 3 atm for 4.5 minutes prior to stent insertion. The balloon was filled with a solution of 166Ho in the irradiation group and with a mixture of contrast media (Visipaque™ 270, Amersham health, Cork, Ireland) and saline (1:1) in the control group, respectively. The irradiation source and its dosage were already described in the previous section.
A 9-Fr sheath was advanced into the portal vein following the slightly deflated balloon catheter. Finally, a nitinol self-expandable stent (10 mm in diameter, 7 cm in length, Niti-S TIPS stent, TaeWoong Medical Co., Koyang, Korea), was placed in the parenchymal tract. The stent position was intended to extend a short distance (about 1 cm) beyond the hepatic vein ostium into the inferior vena cava in the cranial direction and approximately 1-2 cm or more into the portal vein in the caudal direction. The stents were successfully expanded without additional balloon dilatation. Measurements of portal pressure and portography were performed again immediately after stent deployment.
All 11 animals, except one pig that died of peritoneal hemorrhage, tolerated the procedures well during the follow-up period. The TIPS patency of the eleven cases was evaluated using follow-up portal venography and histological analysis two weeks after the procedures.
The animals were then tranquilized by an intramuscular injection of 2 mL ketamine hydrochloride and 2 mL xylazine hydrochloride without continuous intravenous injection. Portal venography was obtained by introducting a 5-Fr catheter into the portal vein via the jugular approach. In two cases, in which the craniocaudal passage of the catheter was disturbed by occlusion of the parenchymal tract and the caudal part (portal vein portion) could not be visualized, portal venography was performed via the superior mesenteric vein that was exposed after an abdominal wall incision (Fig. 3A). The extent and degree of stent narrowing were assessed by comparing the diameters of the stents immediately after and two weeks after TIPS creation. Shunt occlusion was defined as the absence of flow through the shunt, while shunt stenosis was defined as narrowing of at least 50% of the stent diameter.
The animals were then euthanized by an intravenous injection of pentobarbital sodium. The TIPS shunt with a 2 cm rim of surrounding parenchymal tissue was cored out and fixed in formalin solution for two weeks. Thereafter, the stent were sectioned longitudinally at 5 mm thickness intervals. The stent wires were carefully removed with use of a dissecting microscope to minimize damage to the underlying tissue. The cut specimens were processed in paraffin and then transverse sections were obtained perpendicular to the long axis of stent at each level of the portal vein, the parenchymal tract and the hepatic vein. Hematoxylin-eosin (H-E) and Masson-trichrome staining methods were used. On the histological examinations, the granulation tissue from the stent to the luminal surface was defined as the pseudointimal hyperplasia and its maximum thickness was calculated for each animal as a percentage of the estimated stent diameter. The histologic characterization of the pseudointimal hyperplasia was also evaluated.
For comparing the maximal PIH between the two groups, the statistical analysis was performed with using SPSS version 10.0 and two-sample t-tests; statistical significance was set at p values < 0.01. Additionally, the mean portal pressures measured in each pig before and after NBCA embolization and also after TIPS stent insertion were compared in order to confirm the successful creation of portal hypertension and TIPS placement.
Transjugular intrahepatic portosystemic shunt were successfully deployed in all pigs, except in one case of postprocedural death in the control group a day after TIPS creation. The cause of death was intraperitoneal bleeding, and this pig was excluded from the follow-up study.
On follow-up venography, all of the five irradiated stents were patent (5/5, 100%) (Fig. 1A). Meanwhile, the 5 TIPS in the control group were completely occluded (5/6, 83.3%) (Fig. 2A) while the remaining TIPS was partially occluded (1/6, 16.7%) (Fig. 3A). All of shunt stenoses or occlusions developed in the hepatic parenchymal tract of TIPS, while both the hepatic vein and portal vein portions of the TIPS stents had relatively well-preserved lumens.
On histological analysis, the gross specimens in all cases demonstrated partial or complete covering of the stent by glistening white tissue (Fig. 2B). On portal venography, the predominant site of stenosis was within the parenchymal tract. Under microscopic examination, the granulation tissue was proved to be PIH, which was composed of numerous spindle-shaped myofibroblasts, extracellular collagen matrix, undifferentiated mesenchymal cells and inflammatory cells surrounding the stent wires (Figs. 1B, 2C, 2D, 3B). The specimens stained with both H-E and Masson-trichrome revealed that the layers of pseudointimal hyperplasia were sharply demarcated from the surrounding hepatic parenchymal tissue layer. The adjacent hepatic parenchyma appeared normal, except for minimal protrusion into the luminal surface. Masson-trichrome staining confirmed that the intercellular matrix within the PIH was predominately composed of collagen. In addition, a single cell layer that mimicked endothelial cells was below various amounts of fibrin thrombus deposits, which was the most superficial layer and this was seen on the luminal surface. These findings showed similarities with the PIH that occurred in human cases (3, 4). The low-powered magnified histologic specimens obtained from the irradiated stents revealed a thinner and more uniform layer of hyperplasia (Fig. 1B) than that in the nonirradiated control group (Table 2). No other histologic difference, such as the composition and order of arrangement, was evident between the two groups.
Microscopic transverse sections through the portal or hepatic veins showed that the shunt lumen was continuously lined with a single layer of endothelial cell. Proliferation of smooth muscle cells was also noted between the stent and luminal surface, and this represented intimal hyperplasia. Compared with the PIH at the parenchymal level, the intimal hyperplasia at the venous levels had insignificant thickness and it was smooth in contour. No bile staining or bile duct injury was seen in any of the obtained specimens.
The average maximum PIH, which was estimated as a percentage of luminal stenosis per stent radius, was 32.8±13.27% in the irradiated group and 76.0±5.29% in the control group, respectively. Two-sample t-test analysis of the PIH in the irradiated versus the nonirradiated stents demonstrated a statistically significant difference in the maximum thickness of the PIH (p < 0.001).
The mean portal pressure estimated during the procedure was 13.15±3.41 cmH2O and 26.78±5.80 cmH2O before and after the injection of NCBA, respectively (mean increase = 13.63 cmH2O), and this immediately dropped back to 15.76 cmH2O after TIPS creation (mean reduction = 11.02 cmH2O), demonstrating the successful induction of portal hypertension by NCBA embolization, and this was followed by satisfactory reduction of the elevated portal pressure after stent insertion with statistical significance (t-test, p < 0.01).
Irradiation therapy has been reported to be very effective in preventing and reducing neointimal hyperplasia following coronary or noncoronary arterial intervention. The rationale for the use of irradiation to inhibit neointimal hyperplasia is the high susceptibility of actively proliferating cells to the lethal effects of ionizing radiation. Experimental studies have shown that external irradiation or low-dose (12-20 Gy) endovascular irradiation from various sources, i.e., high energy γorβ emitters such as Iridium-192 (192Ir) (6), Yttrium-90 (90Y) (7), Phosphorus-32 (32P) (8) or 166Ho (9), for an arterial stent during or after angioplasty could significantly reduce the arterial myointimal hyperplasia and as a result, increase the original luminal patency. Our experimental study, based on the similar proliferating processes between neointimal hyperplasia and PIH, showed that the intraluminal irradiation using 30 Gy 166Ho during TIPS procedures significantly reduced PIH in a swine model.
The standard local radiation dosages for inhibiting the proliferative process in various settings have not been established. In a previous experimental study with the various activity levels (0.15-23 µCi) of 32P, the doserelated effects of the radiation source showed differential response to the doses of continuous irradiation (the intermediate activity level showed a more severe neointimal response and greater luminal narrowing than that in the nonradioactive stents), that is, a non-linear relation, and this suggested a complex biological interaction of endovascular radiation and vascular repair after stent placement (10). Furthermore, the excess dosage of irradiation is assumed to lead to adverse effects such as extensive parenchymal injury as well as aggravation of thrombosis by the increased fibrin deposits (14). In this experiment, an absorbed radiation dose at the luminal surface of 30 Gy with 4.5 minutes of dwell time was used, and this radiation dose was thought to be a clinically applicable maximum therapeutic dosage for vascular irradiation therapy (15, 16). This dosage was higher than the common dosage (less than 20 Gy) used in neointimal hyperplasia (7, 9, 11). Consequently, the use of this protocol did not induce the radiation-associated changes such as endothelial proliferation or atypical fibroblasts in the vascular structures in any cases, indicating there was acceptable tissue compatibility to a high dosage of 166Ho.
An ideal isotope for brachytherapy should have a high specific activity with short range for avoiding unnecessary irradiation to the adjacent normal tissue, and also achieving a uniform dose distribution over the treated area is a must. To date, 32P has been the most frequently used radionuclide due to its relatively short half-time of 14.3 days, its limited range of 3-4 mm in tissue and no gamma radiation (8, 11). In comparison with 32P, the 166Ho used in this trial is another beta emitter with a specific activity of 7.4 GBq·mg-1, a short physical half-life of 26.8 hours and a short (1-2 mm) range of the absorbed β-particles distribution. These data suggest that 166Ho is superior to 32P from the view of both the physical half-life and the activity range. A few recent studies (9, 16, 17) that were performed in vitro or in vivo with using 166Ho as the radiation source supported this suggestion. Joh et al. (16) showed the 166Ho administered through a balloon angiocatheter in a tissue equivalent solid water phantom delivered an adequate dose within a clinically allowable dwell time, and so this avoided unnecessary irradiation of the normal tissue. Hong et al. (17) demonstrated that 166Ho-DTPA in vivo showed simple radiolabelling, high radiochemical stability (> 98%) and rapid urinary excretion. Kim et al. (9) delivered 20 Gy of 166Ho in a porcine coronary stent restenosis model and the irradiated group demonstrated a significantly decreased stenosing area compared to the control group.
Pig is highly useful as an animal model for evaluating new techniques related to human TIPS, and pigs have been used in the previous related studies (18-20). Further, the liver vascular anatomy of pig is similar with human. Also, PIH in a swine model demonstrated remarkable similarities in the histologic components and development sites to that of human. On the other hand, to mimic the human hemodynamic situations and to create the chronic portal hypertension in animal models, various embolic agents such as gelatin foam, polyvinyl alcohol, stainless steel coils and ethiodized oil have been applied (18, 20, 21). Unfortunately, chronic portal hypertension was successfully created in a dog model only, not in a swine model, in which the increased portal pressure was transient and not prolonged (20, 21). In our study, the portal hypertension was artificially induced via intraportal injection of a NBCA and lipiodol mixture (1:3); this is one of the most common embolic materials used in other various procedures, including treating vascular malformations and prior to lobectomy (22, 23), and administering this material was done in a single session. Our previous pilot study that used two pigs was performed to evaluate the portal pressure after NBCA embolization, and the results showed that the portal hypertension was maintained for two weeks. Despite of the small number of subjects as a limitation, from the results of this pilot study, we can propose the possibility of NBCA embolization for creating stable portal hypertension in a swine model. Based on the pilot study, all the animals of this experiment were assumed to maintain increased peripheral portal venous pressure, and this resulted in preferential blood flow into the TIPS tract rather than into hepatic sinusoids, at least for two weeks.
The follow-up period in this study was determined as two weeks because the pig model provided a highly accelerated rate of shunt stenosis within two to four weeks after TIPS creation when compared to human (shunt stenosis developed two weeks later in human) according to Kichikawa et al. (18) and Lessie et al. (11). In our study, the more rapid development of PIH in the control group (within 2 weeks) may be due to that anticoagulation therapy wasn't given, the stent characteristics and the active regenerative capacity related to young healthy swines, as well as the NBCA, which has known to eventually induce parenchymal hypertrophy (23).
In this study, most of the stenoses were within the parenchymal tract, but TIPS stenoses have actually occurred in both hepatic veins and the parenchymal tracts in clinical practices. This may be explained that the main cause of TIPS stenoses in this study was PIH within the hepatic parenchymal tract, and there was neither thrombosis nor intimal hyperplasia within the vascular sites.
Our study has several limitations. First, the small and uneven sample sizes of the control and irradiated groups and short term follow-up period make it somewhat difficult to conclude the value of 166Ho in TIPS. The two weeks follow-up period is not sufficient to induce cirrhotic changes following the portal hypertension induced by NBCA embolization, even though the portal hypertension was sustained during the experimental period. Second, the concrete evidence for the persistence of portal venous hypertension was not sufficient. In our study, we found that the follow-up portography demonstrated the reconstitution of portal flow within the occluded portal vein in one branch of five pigs. However, active evaluation of the distal branches and collaterals was not performed. Liver function tests, histologic confirmation of the parenchymal tissue and long term follow-up of portal venous pressure are needed to confirm the successful creation of chronic portal venous hypertension in further studies. Third, compared with the human cirrhotic liver, our animal model still has a parenchymal difference such as periportal fibrosis, although the artificial induction of portal hypertension by embolic materials was done to mimic the hemodynamic situation of cirrhosis. This may be followed by the differences of response to radiation, biologic interaction of repair and regeneration, and the stenosis rates.
In conclusion, our study demonstrated a promising result that the intraluminal irradiation using 30 Gy 166Ho during a TIPS procedure significantly reduced PIH in a swine model of portal hypertension and this treatment improved TIPS patency. However, the further studies are certainly required to conclude the clinical feasibility of intraluminal irradiation.
Figures and Tables
Fig. 1
Follow-up studies done two weeks after TIPS with 166Ho irradiation.
A. Portography demonstrates good patency of the shunt without narrowing or occlusion.
B. Microscopic examination (Masson-trichrome stain, ×10) of the specimen obtained from the parenchymal tract of the patent stent reveals that the luminal surface is lined by a relatively thin, uniform layer of pseudointimal hyperplasia. Pseudointimal hyperplasia (H) is well delineated from the normal liver parenchymal layer (nl).
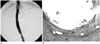
Fig. 2
Follow-up studies done two weeks after TIPS without 166Ho irradiation in the control group.
A. Portography via the transjugular approach demonstrates the complete occlusion of the shunt.
B. The corresponding gross specimen before removal of the stent wire shows marked ingrowth of fibrotic tissue (arrow) within the parenchymal tract, which is in contrast to the lack of significant narrowing at both venous ends (H, hepatic venous end; P, portal venous end).
C. Transverse section (Masson-trichrome stain, ×10) through the parenchymal tract of TIPS reveals the complete luminal occlusion with the most superficial layer of fibrin (F), thick granulation tissue and the inflammatory reaction. The removed portions of the wires (arrowheads) are seen as holes. These stent holes are seen adjacent to the normal parenchymal tissue (nl), which is clearly demarcated from the PIH.
D. Close-up (Masson-trichrome stain, ×100) of the PIH shows numerous spindle-shaped myofibroblasts (arrows) and the abundant collagen matrix.
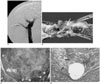
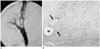
Fig. 3
Follow-up studies done two weeks after TIPS without 166Ho irradiation in the control group.
A. Portography via the superior mesenteric vein, which was exposed by a skin incision, shows partial occlusion of the shunt at the level of parenchymal tract of TIPS.
B. Microscopic cross section revealed a homogeneous layer of granulation tissue that's composed of an abundant acellular matrix, myofibroblasts and numerous inflammatory cells (between arrows) adjacent the stent wire (w).
References
1. Saxon RS, Ross PL, Mendel-Hartvig J, Barton RE, Benner K, Flora K, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998. 207:683–693.
2. Haskal ZJ, Pentecost MJ, Soulen MC, Shlansky-Goldberg RD, Baum RA, Cope C. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994. 163:439–444.
3. Terayama N, Matsui O, Kadoya M, Yoshikawa J, Gabata T, Miyayama S, et al. Transjugular intrahepatic portosystemic shunt: histologic and immunohistochemical study of autopsy cases. Cardiovasc Intervent Radiol. 1997. 20:457–461.
4. Ducoin H, El-Khoury J, Rousseau H, Barange K, Peron JM, Pierragi MT, et al. Histopathologic analysis of transjugular intrahepatic portosystemic shunts. Hepatology. 1997. 25:1064–1069.
5. Teng GJ, Bettmann MA, Hoopes PJ, Wagner RJ, Park BH, Yang L, et al. Transjugular intrahepatic portosystemic shunt: effect of bile leak on smooth muscle cell proliferation. Radiology. 1998. 209:799–805.
6. Condado JA, Waksman R, Calderas C, Saucedo J, Lansky A. Two-year follow-up after intracoronary gamma radiation therapy. Cardiovasc Radiat Med. 1999. 1:30–35.
7. Verin V, Urban P, Popowski Y, Schwager M, Nouet P, Dorsaz PA, et al. Feasibility of intracoronary beta-irradiation to reduce restenosis after balloon angioplasty. A clinical pilot study. Circulation. 1997. 95:1138–1144.
8. Wardeh AJ, Kay IP, Sabate M, Coen VL, Gijzel AL, Ligthart JM, et al. Beta-particle-emitting radioactive stent implantation. A safety and feasibility study. Circulation. 1999. 100:1684–1689.
9. Kim W, Jeong MH, Park OY, Rhew JY, Bom HS, Choi SJ, et al. Effects of beta-radiation using a holmium-166 coated balloon on neointimal hyperplasia in a porcine coronary stent restenosis model. Circ J. 2003. 67:625–629.
10. Carter AJ, Laird JR, Bailey LR, Hoopes TG, Farb A, Fischell DR, et al. Effects of endovascular radiation from a beta-particle-emitting stent in a porcine coronary restenosis model. A dose-response study. Circulation. 1996. 94:2364–2368.
11. Lessie T, Yoon HC, Nelson HA, Fillmore DJ, Baldwin GN, Miller FJ. Intraluminal irradiation for TIPS stenosis: preliminary results in a swine model. J Vasc Interv Radiol. 1999. 10:899–906.
12. Rajendran JG, Eary JF, Bensinger W, Durack LD, Vernon C, Fritzberg A. High-dose 166Ho-DOTMP in myeloablative treatment of multiple myeloma: pharmacokinetics, biodistribution, and absorbed dose estimation. J Nucl Med. 2002. 43:1383–1390.
13. Firestone RB. Table of isotopes. 1996. New York: Wiley-Interscience.
14. Klugherz BD, Meneveau NF, Kolansky DM, Herrmann HC, Schiele F, Matthai WH Jr, et al. Predictors of clinical outcome following percutaneous intervention for in-stent restenosis. Am J Cardiol. 2000. 85:1427–1431.
15. Mazur W, Ali MN, Khan MM, Dabaghi SF, DeFelice CA, Paradis P Jr, et al. High dose rate intracoronary radiation for inhibition of neointimal formation in the stented and balloon-injured porcine models of restenosis: angiographic, morphometric, and histopathologic analyses. Int J Radiat Oncol Biol Phys. 1996. 36:777–788.
16. Joh CW, Park CH, Kang HJ, Oh YT, Chun , Kim HS, et al. Measurement of radiation absorbed dose in endovascular Ho-166 brachytherapy using a balloon angio-catheter. Nucl Med Commun. 2000. 21:959–964.
17. Hong YD, Park KB, Jang BS, Choi SJ, Choi SM, Kim YM. Holmium-166-DTPA as a liquid source for endovascular brachytherapy. Nucl Med Biol. 2002. 29:833–839.
18. Kichikawa K, Saxon RR, Nishimine K, Nishida N, Uchida BT. Experimental TIPS with spiral Z-stents in swine with and without induced portal hypertension. Cardiovasc Intervent Radiol. 1997. 20:197–203.
19. Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, et al. Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine. Radiology. 1995. 196:341–347.
20. Pavcnik D, Saxon RR, Kubota Y, Tanihata H, Uchida BT, Corless C, et al. Attempted induction of chronic portal venous hypertension with polyvinyl alcohol particles in swine. J Vasc Interv Radiol. 1997. 8:123–128.
21. Palmaz JC, Garcia F, Sibbitt RR, Tio FO, Kopp DT, Schwesinger W, et al. Expandable intrahepatic portacaval shunt stents in dogs with chronic portal hypertension. AJR Am J Roentgenol. 1986. 147:1251–1254.
22. Song JK, Gobin YP, Duckwiler GR, Murayama Y, Frazee JG, Martin NA, et al. N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae. AJNR Am J Neuroradiol. 2001. 22:40–47.
23. Denys A, Lacombe C, Schneider F, Madoff DC, Doenz F, Qanadli SD, et al. Portal vein embolization with N-butyl cyanoacrylate before partial hepatectomy in patients with hepatocellular carcinoma and underlying cirrhosis or advanced fibrosis. J Vasc Interv Radiol. 2005. 16:1667–1674.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download