Abstract
Objective
To determine the prognostic factors for local recurrence of nodular hepatocellular carcinoma after segmental transarterial chemoembolization.
Materials and Methods
Seventy-four nodular hepatocellular carcinoma tumors ≤ 5 cm were retrospectively analyzed for local recurrence after segmental transarterial chemoembolization using follow-up CT images (median follow-up of 17 months, 4-77 months in range). The tumors were divided into four groups (IA, IB, IIA, and IIB) according to whether the one-month follow-up CT imaging, after segmental transarterial chemoembolization, showed homogeneous (Group I) or inhomogeneous (Group II) iodized oil accumulation, or whether the tumors were located within the liver segment (Group A) or in a segmental border zone (Group B). Comparison of tumor characteristics between Group IA and the other three groups was performed using the chi-square test. Local recurrence rates were compared among the groups using the Kaplan-Meier estimation and log rank test.
Results
Local tumor recurrence occurred in 19 hepatocellular carcinoma tumors (25.7%). There were: 28, 18, 17, and 11 tumors in Group IA, IB, IIA, and IIB, respectively. One of 28 (3.6%) tumors in Group IA, and 18 of 46 (39.1%) tumors in the other three groups showed local recurrence. Comparisons between Group IA and the other three groups showed that the tumor characteristics were similar. One-, two-, and three-year estimated local recurrence rates in Group IA were 0%, 11.1%, and 11.1%, respectively. The difference between Group IA and the other three groups was statistically significant (p = 0.000).
Surgical resection or transplantation has been considered as the gold standard for treatment of hepatocellular carcinoma (HCC) in patients who are surgical candidates (1, 2). However, the surgical indications are usually very limited; consequently transcatheter arterial chemoembolization (TACE) has been widely implemented for the treatment of patients with unresectable HCC (3-8). The prognostic factors for local recurrence of HCC tumors, after segmental TACE, are already well known and include tumor size and homogeneity of iodized oil accumulation (9, 10). However, we recently reported that the tumor location in the segmental border zone was also an important prognostic factor of local tumor recurrence after TACE (11). We found that tumors located in the segmental border zone showed a higher incidence of local recurrence compared to other tumors (11).
In this study, we evaluated the local tumor recurrence rate of HCC, after segmental TACE, by classifying the tumors based on multiple prognostic factors. We also attempted to determine whether a group with clinically favorable outcome could be identified. In addition, we provide practical guidelines for choice of interventional treatment modality in patients with nodular small or intermediate sized tumors.
Requirements for study participation were as follows: (a) an adult patient with hepatic cirrhosis and either a single 5 cm in diameter HCC or smaller, or as many as three 3 cm each in diameter or smaller HCCs, (b) absence of vascular invasion or extrahepatic metastases, (c) sharp definition from the surrounding liver parenchyma without evidence of adjacent satellite nodules, (d) hepatic cirrhosis classified as Child-Pugh class A or B, (e) prothrombin time ratio (i.e., normal time divided by patient's time) greater than 40%, (f) platelet count higher than 40,000 per cubic millimeter (40-109 /L), (g) no previous treatment for HCC, (h) ineligibility for surgical resection or transplantation, (i) patient agreement to have TACE, (j) no residual enhanced area within or around the tumors on the one-month follow-up CT imaging. The reason for the confined inclusion criteria to nodular small or intermediate tumors was to facilitate comparison with the clinical results from other treatment modalities such as radiofrequency (RF) ablation. From July 1998 to November 2005, 372 patients with HCC had been referred to our center for TACE. Among them, 74 tumors in 59 patients were included in our study. The median follow-up period was 17 months (4-77 months). All but two patients were male. Their age ranged from 44 to 78 years (mean±SD: 62.5±9.5 years). The tumor size ranged from 1.0 cm to 4.5 cm (mean±SD: 2.4±0.9 cm). Additional patient characteristics are shown in Table 1.
For nine patients, the diagnosis of HCC was proved histopathologically. For the other patients, the diagnosis was established on the basis of characteristic imaging findings on the three-phase helical computed tomography (CT) and conventional angiography and/or the presence of elevated tumor marker levels in serum (alpha-fetoprotein level > 200 ng/mL) (12). In the vast majority of patients, the etiology of cirrhosis was chronic viral hepatitis B or C (Table 1).
All patients had enhanced dynamic CT within four weeks prior to TACE. All the TACE procedures were performed by interventional radiologists with experience of over five and three years. Hepatic angiography was performed using 5-Fr angiographic catheters, followed by superselection of segmental arterial feeders using a microcatheter. Then we administered an iodized oildoxorubicin hydrochloride (Adriamycin; Kyowa Hakko Kogyo, Tokyo, Japan) emulsion into the feeders. The volume of iodized oil ranged from 3 to 10 ml, and the amount of doxorubin ranged from 20 to 70 mg. Once the flow became sluggish, gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI) that were mixed with mitomycin-C (Kyowa Hakko Kogyo, Tokyo, Japan) and contrast material (Iopromide; Schering, Berlin, Germany) were administered into the feeders until blood flow stopped completely. While performing the segmental TACE, attempts were made to completely occlude the arterial feeder. A small amount of saline solution was then injected slowly to confirm the complete occlusion of the segmental arterial feeder. If contrast media retention was partially washed out after the saline injection, additional gelatin sponge particles were infused until complete stasis of flow was obtained.
Among the 74 tumors, three were supplied by both segmental arteries on selective angiography using microcatheters. In these circumstances, both segmental feeding arteries were embolized.
Local recurrence rate was compared by 12 possible prognostic factors: patient age, hepatitis C infection, modified Child-Pugh classification, number of tumors, size of tumor nodule, serum alpha-fetoprotein level, serum albumin level, platelet count, homogeneity of iodized oil accumulation within the nodule, tumor location in segmental border zone, tumor location in subcapsular area, and contact of tumor with adjacent vessels.
The number of tumors was determined by pre-embolization CT. Tumor size was determined as the maximal diameter of the nodule measured on the pre-embolization CT. The CT examinations were performed with an 8-slice multidetector CT scanner (Lightspeed; GE Medical Systems, Milwaukee, WI) with 5-mm collimation and 17.5-mm/sec table speed, or a single-detector helical scanner (Prospeed Advantage; GE Medical Systems, Milwaukee, WI) with 10-mm collimation and a 10-mm/sec table speed.
The border zone between hepatic segments was determined by tracing the portal venous tree during the portal phase of the spiral CT scan (13, 14). The segmental border zone was defined as an area without traceable portal veins between hepatic segments on the CT scan. Segmental border zone lesions were defined as lesions crossing an imaginary border between hepatic segments.
All patients underwent both non-enhanced and contrast-enhanced three-phase helical CT four weeks after the TACE. The pattern of iodized oil accumulation in the masses was evaluated with the four-week follow-up CT scan. When the tumor nodules showed compact iodized-oil accumulation without any defect, they were classified as a "homogeneous" pattern; otherwise they were classified as an "inhomogenous" pattern.
When the tumor nodules showed sharp definition from the surrounding liver parenchyma, without evidence of adjacent satellite nodules, they were classified as "nodular" tumors. Otherwise, they were classified "nonnodular" tumors.
Tumor location was classified as either subcapsular (abutting the hepatic capsule) or nonsubcapsular. The tumors were also classified into two groups of contacting or non-contacting tumors according to their position relative to adjacent visible (> 1-mm diameter) blood vessels on the basis of whether part of the tumor was attached to the vessel or not (15). These two factors were known as potential predictors of local recurrence after RF ablation (15-18).
Residual viable tumor was determined to be present when an enhanced portion was observed within or around the original mass on the one-month follow-up CT scan. If no definite evidence of residual tumor was noted on this one-month follow-up CT, then a three-phase contrast-enhanced CT was performed at 3- or 4-month intervals thereafter. Local tumor recurrence was determined to be present when iodized oil from the lesion disappeared, or when an enhanced portion was seen within or at the margin of the original mass on the next follow-up CT scan, after the first one-month follow-up CT scan. For recurrent tumors, additional therapies such as TACE or RF ablation were performed.
Two abdominal radiologists with five and four years of experience interpreted the CT images including segmental zonal anatomy independently; they were blinded to whether the tumor showed local recurrence on the follow-up CT images. Final decisions were reached by consensus.
For the 12 potential prognostic factors of local tumor recurrence, univariate and multivariate analyses were performed using the Cox proportional hazard model. Parameters that proved to be significant with the univariate analysis were subsequently tested with the multivariate Cox proportional hazard model.
Specifically, nodular tumors were divided into four groups as IA, IB, IIA, or IIB according to whether they showed homogeneous (Group I) or inhomogeneous (Group II) iodized oil accumulation on the one-month follow-up CT imaging, after segmental TACE, or whether they were located within the liver segment (Group A) or within the segmental border zone (Group B).
Tumor characteristics such as tumor size, iodized oil uptake pattern on the one-month follow-up CT imaging as well as laboratory data were compared between Group IA and the other three groups combined as well as between each group. Comparison of tumor characteristics and laboratory data between the groups was performed using the chi-square test. The local recurrence rate was compared between the groups using the Kaplan-Meier method and the log rank test.
P-values less than 0.05 were considered statistically significant. The SPSS software package (Version 10.0; SPSS Inc., Chicago, IL) was used for statistical analysis.
The median CT follow-up was 17 months, with a range of 4-78 months. Local tumor recurrence occurred in 19 of the 74 nodular HCC tumors (25.7%). Among the 12 possible prognostic factors, the iodized oil uptake pattern within the tumor and the tumor location in the segmental border zone were statistically significant adverse prognostic factors (c = 0.001 and 0.010, respectively). However, other factors such as tumor size, patient age, modified Child-Pugh classification, hepatitis C, or serum alpha-fetoprotein level, serum albumin level, platelet count, tumor location in the subcapsular area, or contact of tumor with adjacent hepatic vessels were not significantly associated with local tumor recurrence (Table 2). Of note was that the tumor location in the subcapsular area and contact of the tumor with adjacent hepatic vessels were not associated with local recurrence (Table 2). The local recurrence rate of intermediate tumors after segmental TACE (4/17; 23.5%) was similar to that of small HCC (15/57; 26.3%). Multivariate analysis revealed that the iodized oil uptake pattern within the tumor and the tumor location within the segmental border zone were statistically significant independent prognostic factors (p = 0.000 and 0.004, respectively).
There were 28, 18, 17 and 11 tumors in Group IA, IB, IIA and IIB, respectively. Local recurrence occurred for 1, 6, 5 and 7 tumors among Group IA, IB, IIA and IIB, respectively. The local recurrence rates of Group IA, IB, IIA and IIB were 3.6%, 33.3%, 29.4% and 63.6%, respectively. The estimated one-year local recurrence rates were 0%, 14.1%, 41.2% and 63.2% for Group IA, IB, IIA and IIB, respectively. For Group IA, the estimated 2-, and 3-year local recurrence rates were 11.1%, and 11.1%, respectively (Figs. 1, 2). For Group IB, the estimated 2-year local recurrence rate was 36.2%. The difference between Group IA and all the other three groups was statistically significant (p = 0.000). The comparison of tumor characteristics and follow-up period between Group IA and the other three Groups altogether is presented in Table 3. Tumor characteristics were similar among the groups (Table 3).
The difference between Group IA and IB and between Group IB and IIB was also statistically significant (p = 0.001 and 0.003, respectively) (Fig. 3). However, the difference between Group IB and IIA or between Group IIA and IIB was not statistically significant (p = 0.600 and 0.146, respectively). The tumor characteristics were also similar among the four groups (IA, IB, IIA, and IIB). The period of local tumor recurrence after segmental TACE ranged from three months to 20 months (median: 10 months). The treatment of the local recurrent tumors included: RF ablation for six tumors, TACE for 11 tumors and surgical resection for one tumor. An additional tumor was conservatively treated because of poor liver function. Eleven of the 17 treated recurrent tumors showed no evidence of residual viable tumor on the follow-up CT scan. The estimated 1-year and 3-year overall survival rates were 92.6% and 48.7%, respectively. At the end of the study, 16 patients expired; among them, four patients died with recurrent HCC.
The prognostic factors for local recurrence of HCC, after segmental TACE, are well known and have been previously described in the medical literature (3-8). Recently, tumor location, in segmental border zone, has been found to be another important prognostic factor (11). In this study we have demonstrated that the inhomogeneous iodized oil accumulation pattern and the tumor location within the segmental border zone were significant adverse prognostic factors for local tumor recurrence of HCC after segmental TACE (11). Therefore, we classified the tumors into four groups according to the two significant prognostic factors identified in this study. Tumor size was not found to be associated with local tumor recurrence. This finding is in contrast to prior studies (9, 10), and may have been due to the fact that only tumors less than or equal to 5 cm were included in this study, and that relatively strict measures of segmental tumor feeder occlusion were applied in most cases.
For Group IA, the recurrence rate was only 3.6%, and the estimated 3-year local recurrence rate was acceptably low (11.1%). The local recurrence rate of HCC, after segmental TACE, reported in prior studies has been around 30% (9). RF ablation therapy has been previously used primarily for the treatment of small nodular HCC tumors (16, 18-22). The local recurrence rate of small HCCs after RF ablation has been reported to be around 10-20% (16, 18-22). Therefore, the 11.1% estimated 3-year local recurrence rate in Group IA can be regarded as acceptably low. The benefit of additional therapy, such as RF ablation, for these tumors is therefore questionable.
Our results suggest that if tumors were located within the liver segment, or a 1-month follow-up CT scan after segmental TACE revealed compact homogeneous iodized oil accumulation within the tumors, then just follow-up with CT imaging would be adequate for local tumor control. Moreover, in this study, the local recurrence rate of intermediate sized tumors after segmental TACE was similar to that of small HCC. However, in many cases it remains challenging to treat intermediate HCC with RF ablation (19, 23). RF ablation has been shown to result in relatively high recurrence rates for tumors in contact with adjacent hepatic vessels (15, 24-27). Segmental TACE can be an effective alternative to RF ablation in these situations, especially for intermediate size tumors or tumors in contact with adjacent intrahepatic vessels. Segmental TACE may therefore provide an effective option in appropriate patients in addition to the palliative treatment modality.
Even when the first segmental TACE did not result in a satisfactory outcome additional therapy, such as RF ablation can be performed shortly after the segmental TACE. In Korea, RF ablation therapy is not usually covered by medical insurance, and this stepwise approach may reduce the economic burden on patients. Further studies are needed to better understand the cost-effectiveness of these procedures.
The findings from this study suggest that the probability of local recurrence, after segmental TACE, can be stratified according to multiple prognostic factors, especially the iodized oil accumulation pattern and the tumor location within the segmental border zone. Significant differences were observed between Group IA and IB, and also between Group IB and IIB. However, there was no statistical difference in local recurrence rates between Group IB and IIA and between Group IIA and IIB. The absence of significance in these groups may have been caused by the small size of the tumors included in these groups. Larger studies will be needed to clarify the statistical difference between these groups.
The limitations of this study were as follows. First, the segmental anatomy was not determined by CT arterioportography. However, the final determination of the segmental anatomy of the tumor was made by consensus of two experienced abdominal radiologists who traced the portal venous tree during the portal phase of the spiral CT scan. Second, a unified helical CT and angiography approach may improve the clinical results of segmental TACE. Third, an enhanced area, on the follow-up CT imaging, might have been masked by overlying iodized oil accumulation in the tumor. Fourth, the number of tumors in each group was relatively small. Finally, no pathological confirmation was obtained for local recurring tumors.
In conclusion, nodular small or intermediate HCC showed different rates of local recurrence, after segmental TACE, according to the iodized oil accumulation pattern and tumor location within the segmental border zone. Nodular small or intermediate tumors located within the liver segment with homogeneous iodized oil accumulation had an excellent prognosis with regard to local recurrence after segmental TACE, making additional therapy for tumor treatment unnecessary.
Figures and Tables
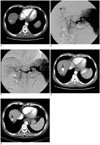 | Fig. 1A 57-year-old male patient with hepatocellular carcinoma.
A. Pre-embolization arterial phase CT image shows a small high density lesion within liver segment 5 (white arrow). Portal phase CT imaging showed washout of the contrast enhancement (not shown). Hepatocellular carcinoma was diagnosed based on the typical CT findings.
B. Arterial phase pre-embolization hepatic angiogram reveals the corresponding tumor with hypervascular staining (arrow).
C. Post-embolization hepatic angiogram shows no evidence of residual tumor staining. Note that the corresponding segmental artery was occluded.
D. One-month follow-up CT imaging, during the arterial phase, shows the mass with iodized oil accumulation (arrow). There was no evidence of a residual enhanced portion within or around the tumor.
E. Follow-up CT imaging, during the arterial phase, 29 months after the segmental transarterial chemoembolization, shows the same mass with reduction in size (arrow). There was no evidence of local tumor recurrence.
|
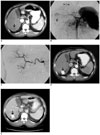 | Fig. 2A 61-year-old male patient with hepatocellular carcinoma.
A. Pre-embolization arterial phase CT imaging shows a small high density lesion located within the segmental border zone between liver segment 6 and 7 (arrow). Portal phase CT imaging showed washout of the contrast enhancement (not shown). Hepatocellular carcinoma was diagnosed based on the typical CT findings.
B. Arterial phase pre-embolization hepatic angiogram reveals the corresponding tumor with hypervascular staining (arrow). The other small hypervascular mass was confirmed as a hemangioma (arrowheads).
C. Post-embolization hepatic angiogram shows no evidence of residual tumor staining. Note that the corresponding segmental artery was occluded.
D. One-month follow-up CT imaging, during the arterial phase, shows the mass with iodized oil accumulation (arrow). There was no evidence of a residual enhanced portion within or around the tumor.
E. Follow-up CT imaging, during the portal phase, four months after the segmental transarterial chemoembolization shows a mass with irregular margin at the same location, highly suggesting local tumor recurrence (arrow). Arterial phase imaging showed subtle contrast enhancement of the lesion (not shown). Hepatic angiogram revealed irregular margined hypervascular tumor staining, and further transarterial chemoembolization was performed.
|
 | Fig. 3Comparison of local tumor recurrence rates between the four groups. Kaplan-Meier estimation and the log rank test were used. The difference between Group IA and IB and between Group IB and IIB was statistically significant (p = 0.001 and 0.003, respectively). All tumors were the nodular type, and the iodized oil accumulation was evaluated at the one-month follow-up CT imaging after transarterial chemoembolization (Group IA: tumors located within liver segment, with homogeneous iodized oil accumulation within the tumors; Group IB: tumors located in segmental border zone, with homogeneous iodized oil accumulation within the tumors; Group IIA: tumors located within liver segment, with inhomogeneous iodized oil accumulation within the tumors; Group IIB: tumors located in segmental border zone, with inhomogeneous iodized oil accumulation within the tumors). |
References
1. Colella G, Bottelli R, De Carlis L, Sansalone CV, Rondinara GF, Alberti A, et al. Hepatocellular carcinoma: comparison between liver transplantation, resective surgery, ethanol injection, and chemoembolization. Transpl Int. 1998. 11:S193–S196.
2. Segawa T, Izawa K, Tsunoda T, Kanematsu T, Shima M, Matsunaga N, et al. Evaluation of hepatectomy in small hepatocellular carcinoma-- comparison with transcatheter arterial embolization therapy. Nippon Geka Gakkai Zasshi. 1992. 93:1095–1099.
3. Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oil chemoembolization of hepatocellular carcinoma. Radiology. 1989. 170:783–786.
4. Bronowicki JP, Vetter D, Dumas F, Boudjema K, Bader R, Weiss AM, et al. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4-year study of 127 French patients. Cancer. 1994. 74:16–24.
5. Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, et al. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990. 13:140–145.
6. Takayasu K, Suzuki M, Uesaka K, Muramatsu Y, Moriyama N, Yoshida T, et al. Hepatic artery embolization for inoperable hepatocellular carcinoma; prognosis and risk factors. Cancer Chemother Pharmacol. 1989. 23:S123–S125.
7. Yamada R, Kishi K, Sonomura T, Tsuda M, Nomura S, Satoh M. Transcatheter arterial embolization in unresectable hepatoellular carcinoma. Cardiovasc Intervent Radiol. 1990. 13:135–139.
8. Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990. 11:181–184.
9. Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001. 176:681–688.
10. Maeda S, Fujiyama S, Tanaka M, Ashihara H, Hirata R, Tomita K. Survival and local recurrence rates of hepatocellular carcinoma patients treated by transarterial chemolipiodolization with and without embolization. Hepatol Res. 2002. 23:202–210.
11. Cho YK, Chung JW, Ahn YS, Park YO, Kim JK, Byun JH. Risk factors for local tumor recurrence after segmental transarterial chemoembolization in hepatocellular carcinoma: the importance of tumor located in the segmental border zone. Korean J Radiol. 2006. 7:267–274.
12. Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002. 36:410–417.
13. Fischer L, Cardenas C, Thorn M, Benner A, Grenacher L, Vetter M, et al. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. J Comput Assist Tomogr. 2002. 26:962–967.
14. Choi D, Choo SW, Lim JH, Lee SJ, Do YS, Choo IW. Opacification of the intrahepatic portal veins during CT hepatic arteriography. J Comput Assist Tomogr. 2001. 25:218–224.
15. Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003. 38:977–981.
16. Cho YK, Rhim H, Ahn YS, Kim MY, Lim HK. Percutaneous radiofrequency ablation therapy of hepatocellular carcinoma using multitined expandable electrodes: comparison of subcapsular and nonsubcapsular tumors. AJR Am J Roentgenol. 2006. 186:S269–S274.
17. Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004. 11:281–289.
18. Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003. 97:1253–1262.
19. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.
20. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocelluar carcinoma in cirrhosis: Randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003. 228:235–240.
21. Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000. 232:381–391.
22. Bonny C, Abergel A, Gayard P, Chouzet S, Ugehetto S, Slim K, et al. Radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis. Gastroenterol Clin Biol. 2002. 26:735–741. (Article in French).
23. Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006. 17:671–683.
24. de Baere T, Bessoud B, Dromain C, Ducreux M, Boige V, Lassau N, et al. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002. 178:53–59.
25. Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002. 95:2353–2260.
26. Rhim H, Kim YS. Intrahepatic regional portal blood flow modulation using percutaneous US-monitoring portal vein compression during radiofrequency thermal ablation. Cardiovasc Intervent Radiol. 2003. 26:416–418.
27. Rhim H. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow: comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2003. 98:433–434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download