Abstract
Objective
We wanted to evaluate the mammographic and sonographic differential features between pure (PT) and mixed tubular carcinoma (MT) of the breast.
Materials and Methods
Between January 1998 and May 2004, 17 PTs and 14 MTs were pathologically confirmed at our institution. The preoperative mammography (n = 26) and sonography (n = 28) were analyzed by three radiologists according to BI-RADS.
Results
On mammography, a mass was not detected in eight patients with PT and in one patient with MT (57% vs. 8%, respectively, p = 0.021), which was statistically different. The other findings on mammography and sonography showed no statistical differences between the PT and MT, although the numerical values were different. When the lesions were detected mammographically, an irregularly shaped mass with a spiculated margin was more frequently found in the MT than in the PT (100% vs. 83%, respectively, p = 0.353). On sonography, all 28 patients presented with a mass and most lesions showed as not being circumscribed, hypoechoic masses with an echogenic halo. Surrounding tissue changes and posterior shadowing were more frequently found in the MT than in the PT (75% vs. 50%, respectively, p = 0.253, 58% vs. 19%, respectively, p = 1.000). An oval shaped mass was more frequently found in the PT than in the MT (44% vs. 25%, respectively; p = 0.434).
Conclusion
PT and MT cannot be precisely differentiated on mammography and sonography. However, the absence of a mass on mammography or the presence of an oval shaped mass would favor the diagnosis of PT. An irregularly shaped mass with surrounding tissue change and posterior shadowing on sonography would favor the diagnosis of MT and also a less favorable prognosis.
Tubular carcinoma of the breast is a special type of breast carcinoma with a particularly favorable prognosis and this tumor is composed of distinct, well-differentiated tubular structures with open lumens that are lined by a single layer of epithelial cells (1). Tubular carcinoma constitutes less than 2% of all breast carcinomas (1, 2), but because they tend to manifest as small lesions, they are found at a higher frequency, i.e., up to 7% in a series of small T1 breast cancers. Tubular carcinomas are often readily detectable mammographically because of their spiculate nature and the associated cellular stroma, and they were seen at higher frequencies of 9-19% in a mammographic screening series (2).
Until 2002, there was a lack of consensus concerning the proportion of tubular structures that's required to pathologically establish the diagnosis of tubular carcinoma. According to the new WHO classification, the diagnosis of pure tubular carcinoma (PT) is made when more than 90% of the tumor exhibits the tubular growth pattern (1). Tumors that are composed of between 50 to 90% of the tubular growth pattern and the other histologic subtypes should be regarded as a mixed type of tubular carcinoma (MT) (1, 2).
Pathologically, Deos and Norris (3) described the differential feature between PTs and MTs. Differentiating PTs from MTs is important because it affects the prognosis. As the percentage of nontubular elements in a tumor increases, so does the likelihood of lymph node metastasis and multifocality (2, 3). A few studies have described the mammographic and sonographic features of tubular carcinoma of the breast (4-6). However, to the best of our knowledge there has been no literature reporting the differential features between the two groups of tubular carcinoma.
The purpose of this study is to evaluate the mammographic and sonographic differential features between PT and MT of the breast.
A computer search of our pathology records revealed a total of 40 cases of pathologically proven tubular carcinomas that were seen at our hospital between January 1998 and May 2004. Those cases with less than 50% of the tubular growth pattern were excluded from this study. As a result, 31 patients (age range: 34-73 years; mean: 47.6 years) with 31 tubular carcinomas were ultimately selected for retrospective analysis. Seventeen patients had PT (age range: 34-73 years; mean: 46.5 years) and 14 patients had MT (age range: 39-68 years; mean: 48.9 years). A mass was classified as palpable if it was felt by the patient, the referring physician or the consulting physician at our institution. A palpable lump was felt in 10 of 17 patients with PT (10/17, 59%) and in eight of 14 patients with MT (8/14, 57%).
Mammograms were available for 26 patients and they were not available for the other five patients who underwent mammography at other hospitals. Mammography in two standard image planes (the mediolateral oblique and craniocaudal views) was performed using Senographe DMR (GE Medical Systems, Milwaukee, WI) or Performa (Instrumentarium Corp, Tuusula, Finland). The mammography was reviewed independently by three radiologists. Any discrepancy in opinion was resolved by consensus. All three radiologists were not informed of the pathologic results. The mammographic findings were evaluated according to the new Breast Imaging Reporting and Data System (BI-RADS) (7): i.e., the presence of a mass, the shape of the mass, the margin characteristics, calcifications, the associated findings and the location and size were evaluated. The central density and length of the spicules were also evaluated. The parenchymal patterns were categorized as fatty (pattern 1), scattered fibroglandular tissue (pattern 2), heterogeneously dense (pattern 3), and extremely dense (pattern 4) with using the BI-RADS.
The sonograms were available for 28 patients with tubular carcinoma, and the sonography was performed using a broad band linear array transducer (5-12 MHz) with a Ultramark 9, a HDI-3000 or a HDI-5000 apparatus (Advanced Technology Laboratories, Bothell, WA). Both the mammography and sonography were performed on the same day for 10 of these 28 patients. In the remaining 18 patients, the mean time between the mammography and sonography was 10.5 days (range: 3-71 days). All the sonographic films were retrospectively reviewed and the sonographic features were recorded independently without any reference to the mammographic and pathologic findings. Again, any discrepancy in opinion was resolved by consensus. When a mass was present, the sonographic findings of the mass were evaluated according to the BI-RADS (7): the shape of the mass, the orientation, the margin of the mass, lesion boundaries, the echo pattern, the posterior acoustic features, the surrounding tissue changes, the presence of calcifications, vascularity, the lymph node involvement and the tumor size were evaluated.
Twenty four preoperative biopsies were performed on 23 patients. Five palpation-guided and five sonography-guided fine needle aspiration biopsies were performed. Fourteen-gauge core needle biopsies were performed under sonographic guidance on 14 patients. One patient underwent core needle biopsy after fine needle aspiration biopsy. Mastectomy with axillary lymph node dissection was performed on 10 patients, and breast conserving surgery with axillary lymph node dissection was performed on 20 of the 31 patients. Excision of the mass without axillary lymph node dissection was performed on the other one patient. All the histologic slides for 31 patients were reviewed by a single pathologist.
The percentage of the distinct tubular growth pattern was calculated in each tumor mass. The tubular carcinomas were then divided into two categories: when the tubular structures composed 90% or greater of the tumor, then it was classified as PT, and when the tubular structures composed between 50 and 90% of the tumor, then it was classified as MT (1). In the 30 cases treated with axillary lymph node dissection, the total number of lymph nodes sampled and the number of nodes with positive findings were recorded. The multifocality, surgical pathologic stage, nuclear grade of the tumor, the tumor size and the associated histology were also assessed.
All the results were analyzed with the SPSS version 10.0 (Statistical Package for the Social Sciences for Windows [Microsoft]). The statistical significance of all the imaging findings and the histologic parameters for the two different histologic groups were calculated using Fisher's exact test. Two-tailed probability values of 0.05 or less were considered significant.
Mammography was available for 14 patients with PT and for 12 patients with MT. The mammographic findings are summarized in Table 1. Seventeen of 26 tubular carcinomas (65%) were visible on mammography. Fifty seven percent (8/14) of the PTs could not be detected on mammography. Of these eight patients, five patients had clinically palpable masses (Fig. 1A), one patient had nipple discharge and the remaining two patients underwent screening mammography. On the other hand, only one of 12 patients with MT (8%) did not show a mass on mammography and this patient had a palpable mass for three months. There was a significant difference regarding the absence of a mass on mammography between the two groups (p = 0.021). There was no significant difference in the parenchymal pattern between the PT and MT cases (p = 0.19). However, all the patients who had a tumor that was not detected on mammography had dense breast tissue such as parenchymal pattern 3 or 4.
Mammographically, both the PT and MT presented as a mass in 17 patients, and these masses were located in the upper outer quadrant (n = 7), the upper portion (n = 5), the upper inner quadrant (n = 3), the outer portion (n = 1), and the lower outer quadrant (n = 1). These masses commonly showed an irregular shape (16/17, 94%) and a spiculated margin (16/17, 94%). An oval shaped mass with an obscured margin was found in only one patient with PT (Fig. 2A). An irregularly shaped mass with a spiculated margin was more frequently found in the MT than in the PT (11/11, 100% vs. 5/6, 83%, respectively, p = 0.353), but no statistical significant difference was found for the mass shape or the margin between the two groups. A long spiculated margin for which the length of the spicule was longer than the longest diameter of the mass was more frequently found in the MT (Fig. 3A) than in the PT (7/11, 64% vs. 2/5, 40%, respectively, p = 0.596). Benign calcifications were found in only two patients with MT (Figs. 3A, B).
The mean diameter of the tumor on mammography was 1.33 cm (range: 1-2.5 cm) for the patients with PT and 1.45 cm (range: 1-2.5 cm) for the patients with MT. No significant difference was noted for the size of the tumors on mammography between the two groups (p = 1.000).
Sonography was available for 16 patients with PT and for 12 patients with MT. The sonographic findings are summarized in Table 1. All 28 patients with tubular carcinoma presented with a mass on sonography. All nine lesions that were not detected on mammography presented as a mass on sonography (Fig. 1B). These patients underwent sonography because of a clinically palpable lump (n = 6), nipple discharge (n = 1), or for screening purposes (n = 2). Most of the masses (27/28, 96%) were not circumscribed, nor were they hypoechoic masses with an echogenic halo, and the masses had either a parallel (15/28, 54%) or non-parallel (13/28, 46%) orientation. An oval shaped mass was more frequently found in the PT (7/16, 44%) than in the MT (3/12, 25%) (Fig. 2B). Sixty four percent (18/28) of the tubular carcinomas presented as irregularly shaped, hypoechoic masses: 56% (9/16) in the patients with PT and 75% (9/12) in the patients with MT. No significant statistical difference was found for the mass shape, margin, echogenecity and orientation between the two groups. Posterior acoustic shadowing and the surrounding tissue change such as Cooper's ligament change and architectural distortion (Fig. 3C) were more frequently found in the MT than in the PT (58% vs. 19%, respectively, p = 1.000, 75% vs. 50%, respectively, p = 0.253), but no significant statistical difference was found between the two groups. Calcifications were found in one patient with PT and in two patients with MT (Fig. 3C).
Six of 26 tumors were evaluated with Doppler study; flow signals were present in four tumors and they were absent in two tumors. Only one patient with PT showed a prominent enlarged lymph node in the ipsilateral axilla; however, no lymph node metastasis was detected in this patient at surgery. The mean diameter of the tumor on sonography was 1.22 cm (range: 0.5-2.3 cm) in the patients with PT and 1.48 cm (range: 0.5-2.9 cm) in the patients with MT. No significant difference was seen for the size of the tumors on sonography between the two groups (p = 0.687).
On the basis of the percentage of the tubular component, 17 (55%) of the 31 tumors were classified as PT; the remaining 14 (45%) of the 31 tumors were classified as MT. On the basis of the nuclear grade, 25 (81%) of the 31 tumors were classified as grade 1, the other six (19%) of the 31 tumors were grade 2, and none of the tumors was classified as grade 3. No significant difference was found for the nuclear grade of the tumors between the two groups.
The maximum diameter of the tumors on pathology ranged from 0.3 to 2.6 cm. The mean diameter of the tumors on pathology was 1.31 cm (range: 0.4-2.3 cm) in the patients with PT and 1.43 cm (range: 0.3-2.6 cm) in the patients with MT. No significant difference was found in the size of the tumors based on the pathology between the two groups (p = 0.456).
The results of the fine needle aspiration biopsy varied from benign findings such as fibroadenoma, low risk lesion and papillary ductal epithelial proliferation to suspicious findings such as high risk lesion and suggestive of ductal carcinoma. Most of the core needle biopsy showed invasive ductal carcinoma (n = 7) and tubular carcinoma (n = 6), whereas only one case resulted in fibrocystic changes.
Axillary lymph node dissection was performed on 30 patients. Lymph node metastases were found in only three (3/17, 18%) of the 17 patients with PT and in all these three patients, only one lymph node was positive. On the other hand, axillary lymph node metastases were found in six (6/14, 43%) of the 14 patients with MT and only one node was positive in three of these patients, but in the other three patients, two, three and ten lymph nodes were involved, respectively. No significant difference was seen for the lymph node metastases between the two groups (p = 0.233).
The associated histologic types in the two groups are summarized in Table 2 and they include malignant tumors such as ductal carcinoma in situ (n = 20), invasive ductal carcinoma (not otherwise specified) (n = 17), cribriform carcinoma (n = 2), lobular carcinoma (n = 2) and benign findings such as fibrocystic changes (n = 11), adenosis (n = 1) and fibroadenomatous mastopathy (n = 1).
Twelve (71%, 12/17) of the 17 patients with PT were classified as stage I and seven (50%, 7/14) of 14 patients with MT were classified as stage I. No significant difference was found in the pathologic stage between the two groups (p = 0.288).
Multifocal tumors were present in only two patients with MT and each of these tumors was about a 0.3 cm-sized invasive tubular carcinoma and lobular carcinoma, respectively, and these were not visible on the preoperative Imaging studies. No significant difference was found for the multifocality between the two groups (p = 0.196).
In the late 1970s and early 1980s, several papers (3, 8-17) on tubular carcinoma were published, mainly in the pathology literature. This type of breast cancer was first described as a distinct entity by Cornil and Ranvier in 1869 (8, 16). Because of their small size, tubular carcinomas are often nonpalpable and they are usually first detected on mammography as a small spiculated mass (4, 17-20). Some cases of tubular carcinoma may be mammographically invisible (6, 17, 18). However, to our knowledge, there has been no previous report regarding the differential imaging findings between PT and MT for making the prediction of a prognosis based on the percentage of the tubular component. Tubular carcinoma typically appears on mammography as a small, spiculated mass that is indistinguishable from other stellate lesions such as radial scars or usual invasive cancers (6, 19, 20). In our series, the absence of a mass or an oval shaped mass would favor the diagnosis of PT, whereas an irregularly shaped mass with a long spiculated margin would more likely favor the diagnosis of MT. Fifty seven percent (8/14) of PTs were not detected on mammography. The possible reasons for the result of our study might be dense breast tissue and the lesions' small size (4). All the tumors that were not detected on mammography were detected on sonography. Therefore, sonography was more sensitive than mammography for detecting tubular carcinoma in women with dense breast tissue.
Sheppard et al. (5) reported that most tubular carcinomas showed as hypoechoic masses with ill-defined margins and posterior acoustic shadowing on sonography. In our series, all the sonography available in the 28 patients successfully demonstrated a mass. The tumors' oval shape would favor the diagnosis of PT, whereas posterior acoustic shadowing and the surrounding tissue change would be more likely result in the diagnosis of MT. In our study, a higher incidence of surrounding tissue change and posterior acoustic shadowing in the patients with MTs seemed to be due to the tendency of early involvement of Cooper's ligaments and the abundant cellular desmoplastic stroma that accompanies the tubular structures in MTs (2).
Although one report suggested that the presence of calcifications was useful for differentiating invasive carcinomas from radial scars (5, 20), another noted that the calcifications might be unrelated to the adjacent mass (6). Suspicious microcalcifications on mammography have been described in 8-9% of the cases of tubular carcinoma (4-6, 17). In our study, the percentage of cases with microcalcifications on mammography was 17%, and only two cases had MT and none had PT. These two cases showed benign-appearing calcifications (Fig. 3).
In general, tubular carcinoma predominantly affects slightly younger women than does breast cancer. The mean age of our patients was 47.6 years, which was comparable to the mean age in one previous report (3) and it was younger than the mean age in other reports (4, 5, 16). The patients in our study with PT were slightly younger than the patients with MT.
Leibman et al. reported that the average mammographic size of tubular carcinomas was 0.8 cm in the patients with nonpalpable lesions and 1.2 cm when the lesions were palpable (4). In our study, the average size of the tubular carcinomas was 1.37 cm, and we did not find a statistically significant difference in tumor size between the MT and PT.
Deos and Norris (3) have reported that axillary lymph node metastases developed in 29% of the women with MT while this developed in only 6% of the PT patients. In our series, the incidence of axillary lymph node metastasis was higher in the MT patients (43%) than in the PT patients (18%) although the difference was not statistically significant.
Our study has several limitations. The major limitations of this study are the retrospective design and the small number of selected patients in each group. With selecting cases from the pathologic database, the tubular carcinomas that had less than 50% tubular features were excluded after pathologic review. Although there were only a small number of patients in each group, the numbers were adequate to perform statistical analysis. Furthermore, because the analysis of the mammographic and sonographc images was done in consensus, data on interobserver variability cannot be provided.
In conclusion, tubular carcinoma commonly presents as an irregularly shaped mass on both mammography and sonography. Fifty seven percent of PTs might not be detected mammographically, probably owing to dense breast tissue and their small size (Fig. 1). PT and MT could not be precisely differentiated on mammography and sonography in our study. However, when there is a small spiculated mass or a palpable abnormality and the mass is diagnosed as tubular carcinoma on biopsy, then the absence of a mass or oval shaped mass would favor the diagnosis of PT. However, the absence of a mass on mammography or an oval shaped mass would favor the diagnosis of PT. An irregularly shaped mass with surrounding tissue change and posterior shadowing on sonography would favor the diagnosis of MT and also a less favorable prognosis.
Figures and Tables
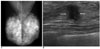 | Fig. 1A 46-year-old woman with pure tubular carcinoma (95% tubular component) with a palpable lump in the right breast.
A. Mammogram shows no definite abnormal focal lesion and both breasts are diffusely dense.
B. Sonography shows an approximately 0.9-cm sized, spiculated, irregularly shaped hypoechoic mass (arrows) in the subareolar area of the right breast.
|
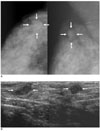 | Fig. 2A 41-year-old woman with pure tubular carcinoma (95% tubular component) with painful palpable lump in the upper outer quadrant of the right breast.
A. Mammography shows an about 1.5-cm sized oval shaped isodense mass with an obscured margin (arrows) in the upper outer quadrant of the right breast.
B. Sonography shows an about 1.2-cm sized oval shaped hypoechoic mass with a microlobulated margin (arrows) in the right breast.
|
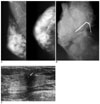 | Fig. 3A 43-year-old woman with mixed tubular carcinoma (70% tubular component) with a palpable lump in the right breast.
A. Mammography shows a long spiculated mass with central lucency (arrows) in the right breast.
B. Specimen mammography shows benign calcifications (circle) in the long spiculated mass.
C. Sonography shows an approximately 1.2-cm sized, irregularly shaped hypoechoic mass with an indistinct margin and calcifications (arrow), and posterior shadowing.
|
Table 1
Comparison of the Mammographic and Sonographic Features of Pure and Mixed Tubular Carcinomas

Note.-*Fisher's exact test for comparison of the imaging features between the two groups with the level of significance set at p < 0.05.
†The number in the parentheses is a percentage and the denominator is the number of total cases with PT (n = 14) or MT (n = 12).
‡The number in the parentheses is a percentage and the denominator is the number of cases with the presence of a mass on mammography.
Acknowledgments
We thank Bonnie Hami, MA, Department of Radiology, University Hospitals Health System, Cleveland, Ohio, for her editorial assistance in the preparation of this manuscript.
References
1. Tavassoli FA, Devilee P. Jaffe ES, Harris NL, Stein H, editors. Pathology and genetics. World Health Organization classification of tumours: tumours of the breast and female genital organs. 2003. Lyon: IARC Press;26–28.
2. Rosen PP. Rosen's breast pathology. 2001. 2nd ed. Philadelphia: Lippincott-Williams & Wilkins;365–380.
3. Deos PH, Norris HJ. Well-differentiated (tubular) carcinoma of the breast. A clinicopathologic study of 145 pure and mixed cases. Am J Clin Pathol. 1982. 78:1–7.
4. Leibman AJ, Lewis M, Kruse B. Tubular carcinoma of the breast: mammographic appearance. AJR Am J Roentgenol. 1993. 160:263–265.
5. Sheppard DG, Whitman GJ, Huynh PT, Sahin AA, Fornage BD, Stelling CB. Tubular carcinoma of the breast: mammographic and sonographic features. AJR Am J Roentgenol. 2000. 174:253–257.
6. Elson BC, Helvie MA, Frank TS, Wilson TE, Adler DD. Tubular carcinoma of the breast: mode of presentation, mammographic appearance, and frequency of nodal metastases. AJR Am J Roentgenol. 1993. 161:1173–1176.
7. American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS). 2003. 4th ed. Reston, VA: American College of Radiology.
8. Carstens PH, Huvos AG, Foote FW Jr, Ashikari R. Tubular carcinoma of the breast: a clinicopathologic study of 35 cases. Am J Clin Pathol. 1972. 58:231–238.
9. Cooper HS, Patchefsky AS, Krall RA. Tubular carcinoma of the breast. Cancer. 1978. 42:2334–2342.
10. Oberman HA, Fidler WJ Jr. Tubular carcinoma of the breast. Am J Surg Pathol. 1979. 3:387–395.
11. Peters GN, Wolff M, Haagensen CD. Tubular carcinoma of the breast. Clinical pathologic correlations based on 100 cases. Ann Surg. 1981. 193:138–149.
12. Carstens PH, Greenberg RA, Francis D, Lyon H. Tubular carcinoma of the breast. A long term follow-up. Histopathology. 1985. 9:271–280.
13. Carstens PH. Tubular carcinoma of the breast. A study of frequency. Am J Clin Pathol. 1978. 70:204–210.
14. McDivitt RW, Boyce W, Gersell D. Tubular carcinoma of the breast. Clinical and pathological observations concerning 135 cases. Am J Surg Pathol. 1982. 6:401–411.
15. Lagios MD, Rose MR, Margolin FR. Tubular carcinoma of the breast: association with multicentricity, bilaterality, and family history of mammary carcinoma. Am J Clin Pathol. 1980. 73:25–30.
16. Mitnick JS, Gianutsos R, Pollack AH, Susman M, Baskin BL, Ko WD, et al. Tubular carcinoma of the breast: sensitivity of diagnostic techniques and correlation with histopathology. AJR Am J Roentgenol. 1999. 172:319–323.
17. Feig SA, Shaber GS, Patchefsky AS, Schwartz GF, Edeiken J, Nerlinger R. Tubular carcinoma of the breast. Mammographic appearance and pathological correlation. Radiology. 1978. 129:311–314.
18. McBoyle MF, Razek HA, Carter JL, Helmer SD. Tubular carcinoma of the breast: an institutional review. Am Surg. 1997. 63:639–644.
19. Vega A, Garijo F. Radial scar and tubular carcinoma. Mammographic and sonographic findings. Acta Radiol. 1993. 34:43–47.
20. Mitnick JS, Vazquez MF, Harris MN, Roses DF. Differentiation of radial scar from scirrhous carcinoma of the breast: mammographic-pathologic correlation. Radiology. 1989. 173:697–700.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download