Abstract
Objective
To compare the effect of radio frequency ablation (RFA) on the dimensions of radio frequency coagulation necrosis in a rabbit lung using a wet electrode in monopolar mode with that in dual electrode bipolar mode at different infusion rates (15 mm/hr versus 30 ml/hr) and saline concentrations (0.9% normal versus 5.8% hypertonic saline).
Materials and Methods
Fifty ablation zones (one ablation zone in each rabbit) were produced in 50 rabbits using one or two 16-guage wet electrodes with a 1-cm active tip. The RFA system used in the monopolar and dual electrode wet bipolar RFA consisted of a 375-kHz generator (Elektrotom HiTT 106, Berchtold, Medizinelektronik, Germany). The power used was 30 watts and the exposure time was 5 minutes. The rabbits were assigned to one of five groups. Group A (n = 10) was infused with 0.9% NaCl used at a rate of 30 ml/hr in a monopolar mode. Groups B (n = 10) and C (n = 10) were infused with 0.9% NaCl at a rate of 15 and 30 ml/hr, respectively in dual electrode bipolar mode; groups D (n = 10) and E (n = 10) were infused with 5.8% NaCl at a rate of 15 and 30 ml/hr, respectively in a dual electrode bipolar mode. The dimensions of the ablation zones in the gross specimens from the groups were compared using one-way analysis of variance by means of the Scheffe test (post-hoc testing).
Results
The mean largest diameter of the ablation zones was larger in dual electrode bipolar mode (30.9±4.4 mm) than in monopolar mode (22.5±3.5 mm). The mean smallest diameter of the ablation zones was larger in dual electrode bipolar mode (22.3±2.5 mm) than in monopolar mode (19.5±3.5 mm). There were significant differences in the largest and smallest dimension between the monopolar (group A) and dual electrode wet bipolar mode (groups B-E). In dual electrode bipolar mode, the mean largest diameter of the ablation zones was larger at an infusion rate of 15 ml/hr (34.2±4.0 mm) than at 30 ml/hr (27.6±0.1 mm), and the mean smallest diameter of the ablation zones was larger at an infusion rate of 15 ml/hr (27.2±7.5 mm) than at an infusion rate of 30 ml/hr (24±2.9 mm).
Radio frequency thermal ablation (RFA) can offer physicians a minimally invasive method for treating patients with inoperable lung cancer. Most reports on this subject have demonstrated its feasibility, early results in inducing complete necrosis, and complications in patients with medically or surgically inoperable lung cancer (1-7).
Equipment from RITA Medical Systems (StarBurst XL, CA) and Radionics (CC-1; Radionics, Burlington, MA) are currently available for RFA of lung malignancies. However, the major limitation of these systems is that it is very difficult to create an ablation zone greater than 3 cm diameter that includes the pulmonary malignant mass and a safety margin of healthy tissue (5-10 mm) in a single session. Jin et al. (1) reported that RFA using an internally cooled electrode produced complete ablation for only stage I lung cancer or a metastasis (below 3 cm). Multiple repositioning of the electrode (average, two) was needed if the tumor diameter was greater than 3 cm. Belfiore et al. (4) also reported multiple repositioning (average, two) in 19 out of 33 patients to ensure ablation of the entire mass (1-7).
New approaches have recently been proposed to overcome the limitations of these RFA systems including RFA using a monopolar or bipolar wet electrode. These techniques infuse saline into the tissue through a monopolar or bipolar electrode. Continuous saline infusion through a monopolar electrode can increase the ablation zone because it increases the electrical and thermal conductivity. However, the areas of coagulation are sometimes irregular as a result of the uneven diffusion of saline and heated saline. Compared with the monopolar wet electrode, superior performance of RFA has been shown using a bipolar wet electrode with the formation of coagulation necrosis (8-12). Most studies of RFA using a wet electrode have used hepatic tissue either in vitro or in vivo . However, Lee et al. (9) reported that saline-enhanced RFA of a rabbit lung produces more extensive coagulation necrosis than conventional RFA procedures.
This study compare the effect of monopolar mode with that of dual electrode bipolar mode at different infusion rates (15 ml/hr versus 30 ml/hr) and saline concentrations (0.9% normal versus 5.8% hypertonic saline) on the dimensions of radio frequency (RF) coagulation necrosis in a rabbit lung using a wet electrode.
All the experimental animals used in this study were treated according to the protocol provided by the Institutional Animal Care and Use Committee at our hospital. Fifty New Zealand rabbits weighing 2-3 kg each were anesthetized by an intramuscular injection of 50 mg/kg ketamine hydrochloride (Ketamine; Yuhan, Seoul, Korea) and 5 mg/kg xylazine (Rumpun; Bayer Korea, Ansan, Korea) before the procedures.
The upper back areas were then shaved and sterilized, and the rabbits were positioned prone on the CT table. Axial CT scans (Sensation 16; Siemens, Erlangen, Germany) were obtained with a 1 mm slice thickness and a 0.75 pitch. The CT images included both lower lobes of the lung.
The rabbits were assigned to one of five groups. Group A (n = 10) was infused with 0.9% NaCl at a rate 30 ml/hr with RFA being performed in monopolar mode. Group B (n = 10) was infused with 0.9% NaCl at a rate 30 ml/hr with RFA being performed in dual electrode wet bipolar mode. Group C (n = 10) was infused with 0.9% NaCl at a rate 15 ml/hr with being RFA performed in dual electrode wet bipolar mode. Group D (n = 10) was infused with 5.8% NaCl at a rate 30 ml/hr with RFA being performed in dual electrode wet bipolar mode. Group E (n = 10) was infused with 5.8% NaCl at a rate 15 ml/hr with RFA being performed in dual electrode wet bipolar mode.
The RFA system used for the monopolar and dual electrode wet bipolar RFA consisted of a 375-kHz generator (Elektrotom HiTT 106, Berchtold, Medizinelektronik, Germany). The power applied was 30 watts, and one or two 16-gauge wet electrodes with each electrode being in the form of a needle with a tip exposure of 1 cm were used. Either a 0.9% or 5.8% NaCl solution was used as the perfusion liquid. According to company recommendations, RF energy was applied for 5 minutes and the RF was turned off for 2-min intervals (13). The RF power could be stabilized using the control mechanism if the change in impedance was within the range of 100 to 350Ω. For monopolar RFA, one grounding pad was palced on the abdomen. A 16-gauge single wet electrode (Elektrotom HiTT 106, Berchtold, Medizinelektronik, Germany) was then introduced into the right lower lobe of the rabbit's lung under CT. Two electrodes were used for the dual electrode wet bipolar RFA procedures. The distance between the two electrodes was selected before inserting the needle using an acrylic-blocking device containing multiple holes at 5-mm intervals. This gave operational mobility to the required position. Two electrodes were placed in the lung, 1 cm apart, through an acryl plate. The 0.9% or 5.8% hypertonic saline was infused at a rate of 15 or 30 mL/hr through a wet electrode using an infusion pump (Pilotec IS; Frensenius Vial SA, Breanzins, France). The technical parameters of RFA including the impedance and power changes, the tissue temperature at the midpoint between the two electrodes and within the monopolar electrode measured using a thermocouple, and the dimensions of the ablation zone with each technique were compared. Non-contrast axial CT scans (1 mm slice thickness and a 0.75 pitch) were obtained to determine the morphological change in the ablation zone immediately after RFA. The images were viewed with a lung window setting that was appropriate for the pulmonary parenchyma (window width, 1,500 HU; window center, -750 HU).
All CT images were archived using a picture archiving and communication system (PACS, m-view TM; Marotech, Korea). The consolidation near the electrode and the peripheral ground glass opacity (GGO) on the CT images was evaluated. The dimensions of each consolidation were calculated for all sections where the maximum and minimal diameter of the consolidation was believed to exist. The mean value was taken after measuring the size of each section.
As soon as the RFA procedures were complete, the rabbits were sacrificed by an overdose of Ketamine and Xylazine. The thoracic cage of the sacrificed rabbits was opened immediately and the amount of pleural effusion was checked using a syringe. The rabbits' lungs were then harvested. The gross specimens were fixed in 10% formalin for a routine histology examination.
The macroscopic measurements were performed independently by two investigators. The shapes of the ablation zone were checked after the ablation zone had been dissected along the axis that the electrode had been inserted. The shape of the ablation zone was defined as being spherical if it had a ball-shape in either monopolar or bipolar mode. The shape of the ablation zone was defined as tumbling doll if it had a ball-shape and within two different two ablation sizes in bipolar mode. The two observers then measured the axial diameter (Da) along the probes within the ablation zone, and the transverse diameter (Dt) perpendicular to Da. Using two electrodes, the Da and Dt of the each ablation zone was measured and the Da and Dt were added (10). Final processing of the tissue for the optical microscopy study involved paraffin sectioning and hematoxylin-eosin (HE) staining, which was carried out according to the standard protocol. The main interest was in observing the microscopic findings of the coagulate necrosis. A histology study was performed by a pathologist, and the gross analysis was compared with the histology findings.
The dimensions of the ablation zone between the dual electrode wet bipolar and monopolar RFA procedures were compared using one-way analysis of variance by means of the Scheffe test (post-hoc testing) with SPSS 9.0 computer software (SPSS Inc., Chicago, IL) In addition, the Pearson's correlation coefficient was used to correlate the ablation size with the saline concentration, flow rate, tissue temperature and pleural effusion. The values are expressed as a means±SD. For all statistical analyses, p values < 0.05 were considered significant.
Both monopolar and dual electrode wet bipolar probes were used to apply the RF energy, and the tissue impedance increased to more than 150Ω on 2-3 minutes after starting the RFA.
The non-contrast CT scans after RFA showed a circular or ellipsoidal consolidation with an irregular or ill-defined margin near the electrode (central ablation zone) and extensive peripheral GGO (peripheral ablation zone). The mean dimensions of the consolidation near each electrode in groups A, C and E were larger than in groups B and D. The mean maximum diameter and minimum diameter using 5.8% and 0.9% saline were 16.2±2.5 mm and 12.5±4.3 mm, 11.2±3.9 mm and 9±1.7 mm, respectively (Figs. 1A, B and 2A, B). However, the dimension of the peripheral GGO could not be evaluated because the CT scans showed extensive opacity in the entire lower lobe regardless of the saline concentration and infusion rate.
The mean Da values of the RF induced central white zone measured in the gross specimens of groups A, B, C, D, and E were 22.5±3.5 mm, 27.5±6.9 mm, 31.3±6.9 mm, 27.7±3.1 mm and 37±8.6 mm, respectively. The mean Dt values of groups A, B, C, D, and E were 19.5±3.5 mm, 22.3±2.5 mm, 23±7 mm, 25.7±2.7 mm and 31.3±6.3 mm, respectively. The mean Da diameter of the ablation zones was larger in dual electrode bipolar mode (30.9±4.4 mm) than in monopolar mode (22.5±3.5 mm). The mean Dt diameter of the ablation zones was larger in dual electrode bipolar mode (22.3±2.5 mm) than in the monopolar mode (19.5±3.5 mm). There were statistically significant differences in the Da and Dt values between the monopolar (group A) and dual electrode wet bipolar (groups B E) RFA procedures (Table 1) (Fig. 3).
The ablation size was correlated with the saline concentration, flow rate, tissue temperature and pleural effusion in the dual electrode wet bipolar groups (Table 1). Increasing the NaCl concentration did not significantly increase the ablation size: the Da and Dt using 5.8% and 0.9% saline were 36.2±8.5 mm and 28.5±5.3 mm, 36.2±10.9 mm and 22.7±4.7 mm, respectively. The difference in the Da and Dt values between the groups was also not statistically significant. However, the saline infusion rate had a significant affect on the ablation size: the Da and Dt for a rate of 15 ml/hr and 30 ml/hr were 41.2±10.6 mm and 24±2.8 mm, and 31.2±4.3 mm and 24±2.8 mm, respectively. The highest tissue temperature was observed in group E (68±3.4℃). A slower saline infusion rate resulted in a greater increase in the temperature of the ablation zone. The largest amounts of pleural effusion, which were measured immediately after the RFA procedure was complete, were obtained in group D (12.7±2.5 cc). The amount of the pleural effusion was higher in the groups exposed to the dual electrode wet bipolar probe than in those exposed to the monopolar probe. In addition, the amount of the pleural effusion was higher in the groups given hypertonic saline than in those given normal saline. The difference between the ablation size and temperature (r = 0.74, p = 0.05) in each group (B-E), the saline infusion rate and temperature between a infusion rate of 15 cc/hr and 30 cc/hr (r = -0.92, p = 0.020), and the saline concentration and amount of pleural effusion between those given an infusion of 0.9% normal saline and those given 5.8% hypertonic saline (r = 0.88, p = 0.04) were statistically significant.
There were two shapes of ablation zones observed in this study: a spherical and a tumbling doll shape. The ablation zones groups A, C, and E had a spherical shape and those groups B and D had a tumbling doll shape. The ablation zone in group A was concentrically spherical with three layers. Groups C and E had a spherical ablation zone (Figs. 1C, D) but with severe lung destruction. The central layer showed hemorrhage, which contained many inflammatory cells. The ablation zones of groups B and D had a tumbling doll shape with a central wrist (Figs. 2C, D). The microscopic findings showed that the ablation zones in all groups were composed of four regions: (1) severe destruction of the normal lung structure in the center, which was the site of electrode insertion; (2) complete necrosis of the normal lung parenchyma that maintained an alveolar structure, variable thickening of hyalinized alveolar wall, no infiltration of the inflammatory cell and hemorrhage, and amorphous material within the alveoli; (3) incomplete necrosis of the normal lung parenchyma that maintained an alveolar structure without a hyalinized alveolar wall, no infiltration of the inflammatory cell, weakly eosinophilic infiltration, and proteinous material within the alveoli; (4) a hemorrhagic band containing congested alveoli and vessels, neutrophil infiltration , and dilatation of the alveoli. Groups D and E showed larger ablation zones, the most severe hemorrhage and parenchymal destruction compared with the other groups. The second and third layers were larger and showed severe hemorrhage. In addition, groups B and C showed extensive subpleural damage. However, there was less hemorrhage and coagulation necrosis than in the hypertonic groups.
Substantial improvements in RFA techniques have been proposed to increase the volume of the ablation zone. These improvements include multiprobe arrays, bipolar arrays, injecting a saline solution during RF ablation, internally cooled electrodes, clustered electrodes and the pulsed application of RF (14). With a single cooled electrode, it is not always possible to achieve an adequate surgical margin extending 0.5 to 1 cm beyond the targeted tumor in a single session when the size of the tumor is > 3 cm (1-3, 8, 15, 16). Therefore, multiple overlapping ablations are generally needed to achieve complete ablation of the target tumor. However, this technique can cause a great deal of pain in patients and increase the probability of pneumothoraces (18). One of the newer, more effective approaches to overcome this problem is to infuse a saline solution (10, 11, 17, 18). In addition, several studies (11, 19, 20) have demonstrated that bipolar RFA can induce coagulation necrosis over a larger area than monopolar RFA by increasing the current density between the electrodes.
In this study, the dual electrode wet bipolar mode created a larger ablation zone than the wet monopolar mode (Da: 30.9±4.4 mm and 22.5±3.5 mm, respectively). This agrees with the findings reported elsewhere (11), and can be explained by the high current density between the two electrodes and the thermodynamic effects observed in bipolar mode (21, 22). Heat is trapped between the two electrodes resulting in higher temperatures in bipolar mode than in monopolar mode. Therefore, despite the different impedance and tissue conduction between the liver and lung, the dual electrode wet bipolar mode can create a superior ablation zone in a rabbit lung to that produced in wet monopolar mode.
One strategy to potentially increase the treatment response to RF ablation is to modulate the biological environment of the treated tissues. Several reports (9, 16, 20, 24) have shown increased tissue heating and coagulation during the RF ablation procedure by injecting different saline concentrations into the tissues. Lee et al. (16) suggested that an infusion of a 36% NaCl solution during RF ablation could produce a larger lesion in an ex vivo bovine lung and an in vivo rabbit lung than an infusion of 0.9% NaCl solution. Lobo et al. (24) examined agar phantoms with varying NaCl concentrations and volumes, and reported that the maximum tissue temperature could be achieved with a high NaCl concentration. The NaCl concentration has an influence on both the current density and the heat distribution. In addition, the RF heating of tissue is directly proportional to the current density applied to a tissue. Therefore, the ablation size is proportional to the NaCl concentration. However, the ablation size in the rabbit lung was similar when using 0.9% and 5.8% NaCl solutions. One of the reasons for this might be that a 5.8% NaCl solution does nor significantly increase the tissue tonicity compared with a 36% NaCl solution, which was also reported by Lee et al. (16, 17, 25, 26).
In this study, the ablation size at a given NaCl concentration was significantly larger when using a low flow rate (15 mL/h) than when using a high flow rate (30 mL/h), which concurs with the results reported in the literature (23-25). Lobo et al. (24) reported significant increases in temperature with increasing NaCl volumes at a given NaCl concentration. However, they reported that although the temperature increased to a certain maximum value, any further increases in the NaCl gel volume resulted in a decrease in temperature. This might be because the high infusion rates place a larger amount of solution in the tissue, which decreases the temperature around the RF electrode. This is basically the same cooling phenomenon reported for perfusion-mediated cooling.
There were two shapes of ablation zones in this study, spherical and tumbling doll shapes. The spherical zones were created using the monopolar method and the dual electrode wet bipolar method with a low infusion rate (15 mL/hr). The tumbling doll shaped zones were created using the dual electrode wet bipolar method with a high infusion rate (30 mL/hr). Lee et al. (10) reported that bipolar RFA could create a higher current density between the two electrodes. More extensive, well-defined, RF-induced coagulation necrosis could be induced this way, which can produce a well-defined oval-shaped lesion. However, RFA using a dual bipolar wet electrode created two different ablation zones shapes according to the infusion rate. A high infusion rate might allow for reduced power between the two electrodes and a less desirable deep waist formation in the midpoint between the electrodes. Burdio et al. (27) reported that uneven electric contact between the two electrodes does not have a significant effect on the shape of the ablation zone when the emitted power is low (usually < 40 W), and can produce regular coagulation lesions. In our experience, most coagulation necrosis observed had a regular margin, but unexpected injury was detected around the subpleural area due to saline spillage , which is unlike that observed in the liver. Subpleural parenchymal injury was more severe with the dual bipolar wet electrode than with wet monopolar electrode. Therefore, caution must be taken when using a dual bipolar wet electrode to prevent unexpected subpleural parenchymal and pleural injury to patients with a lung malignancy.
The CT scans obtained immediately after RFA showed central consolidation with extensive GGO. Compared with the histology findings, the central consolidation corresponds to partially complete coagulation necrosis with pulmonary hemorrhage and extensive peripheral GGO corresponding partially complete necrosis, most of the incomplete coagulation necrosis and a pulmonary hemorrhage. The reason for the larger consolidation in the 5.8% hypertonic saline groups than in the 0.9% normal saline groups was that more severe pulmonary hemorrhage occurred within the destroyed tissue and complete coagulation region in the 5.8% hypertonic groups. The peripheral GGO needs to be interpreted from a different viewpoint compared with RFA using dry electrode. Jin et al. (1) reported that peripheral enveloped ground glass opacity > 5 mm is very important for evaluating the complete ablation after RFA using a dry electrode. Although RFA using wet electrode can make an extensive peripheral GGO, an ablation zone within the enveloped GGO does not mean complete coagulation. The histology examination also showed that the incomplete coagulation region was larger in the 5.8% hypertonic groups than in the 0.9% normal saline groups. Therefore, it is possible to overestimate the complete ablation zone in patients with a lung malignancy after RFA using a wet electrode, particularly in those given 5.8% hypertonic saline.
There are reports of complications such as acute pulmonary hemorrhage, pneumothorax, acute respiratory distress syndrome and pleural effusion after RF ablation of the lung tissue in in vivo studies (3, 16). Pleural effusion always occurs after RFA using a wet electrode and three pneumothoraces were experienced in this study. There was a higher incidence of pleural effusion in the group given the higher NaCl concentration than in the group given the lower NaCl concentration. Lee et al. (16) suggested that pleural effusions could occur as a result of spillage of the NaCl solution into the pleural cavity while instilling the solution. More fluid can seep as a pleural effusion via diffusion with a more hypertonic solution in the pleural cavity. Although the amount of the pleural effusion was evaluated 5 minutes after RFA was complete, it is possible that the evaluation time can affect the amount of pleural effusion. In future, it will be important to restrict the amount of the NaCl solution in order to reduce the incidence of pleural effusion after the RFA procedures in human studies. The three pneumothoraces occurred in the bipolar group only. It is possible that the incidence of a pneumothorax will increase with increasing use of the bipolar electrode.
This experimental study had some limitations. First, the rabbit lungs were too small to allow an investigation of the capability of wet electrodes to increase the level of coagulation necrosis. Therefore, the power of RF energy was restricted to low power and the time of saline infusion was shorter than those used clinically. In future, an animal model with a clinically relevant size will be necessary to demonstrate the effectiveness and safety of a wet electrode before it can be used clinically for the treatment of lung malignancies. Second, RFA was not performed using a monopolar electrode with 5.8% hypertonic saline. Therefore, the monopolar electrodes could not be compared directly with the bipolar electrodes using the same hypertonic saline solution. Third, the extent of irreversible cellular injury was not evaluated during the early stages after RFA because the mitochondrial enzyme was not stained with 2% 2,3,5,-triphenyl tetrazolium chloride. Despite this disadvantage, these results provide reliable data for examining the efficacy and safety of the dual wet electrode RFA in creating coagulation necrosis. In clinical aspects, bipolar RFA using a dual electrode may also have some disadvantages compared with monopolar electrodes. First, in bipolar RFA using dual electrodes, the electrodes must be parallel to each other. However, the insertion of two parallel probes can sometimes be difficult to achieve and would demand a longer procedure time. In addition, using two electrodes increases the expense of the RFA procedure. Second, like in this study, when there is a difference in the saline perfusion, one probe might reach a higher temperature than the other, which can lead to incomplete ablation of the lung malignancy.
In conclusion, RFA using a dual bipolar wet electrode is more effective in creating a satisfactory ablation zone than a monopolar electrode in a rabbit lung. However, it is imperative that the optimal infusion rate and the correct NaCl concentration be determined to achieve good results. Further study of this technique in a larger animal model will be needed to determine the effectiveness and safety of this procedure in producing an adequate ablation volume for patients with a lung malignancy.
Figures and Tables
Fig. 1
CT scans and pathology findings of the spherical ablation zones in groups A, C and E.
A, B. Non-contrast CT scans after RFA using the dual wet electrode showed a 15×11 mm, 10×9 mm ellipsoidal consolidation (arrows) with ill-defined margins in the right lower lung field and mild peripheral ground glass opacity.
C, D. Gross specimen (C) shows a central dark area within the ablation zone between the two electrodes (arrow). Microscopic specimen (D) findings shows severe destruction, hemorrhage and complete necrosis of the normal lung structure in the center (arrow). The peripheral region showed incomplete necrosis of the normal lung parenchyma with an alveolar structure maintained without a hyalinized alveolar wall, no infiltration of the inflammatory cell, weak eosinophilic infiltration, and proteinous material within the alveoli (arrowheads).
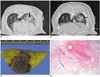
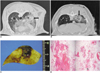
Fig. 2
CT scans and pathology findings of the tumbling doll shape ablation zone in groups B and D.
A, B. The non-contrast CT scans after RFA using a dual wet electrode showed a 12×10 mm , 8×7 circle consolidation (arrows) with an ill-defined margin in right lower lung and extensive peripheral ground-glass opacity.
C, D. The ablation zones show two areas of coagulation necroses. The one electrode (arrow) creats larger ablation zone and the other (arrowhead) creats smaller ablation zone. Microscopic specimens show that larger ablation zone (L in D) has more severe hemorrhage and parenchymal destruction than smaller ablation zone (S in D).
Acknowledgment
The authors thank Mr. Kevin Condren at the Harrisco research institute and Dr. Lee YC who was supported by grants from the National Research Laboratory Program of Korea Science and Engineering Foundation for their editorial assistance in preparing this manuscript. Also, the authors thank Dr. Jang KY who are patholoyist. He helped the authors to prepare the pathologic photography.
References
1. Jin GY, Lee JM, Lee YC, Han YM, Lim YS. Primary and secondary lung malignancies treated with percutaneous radiofrequency ablation: evaluation with follow-up helical CT. AJR Am J Roentgenol. 2004. 183:1013–1020.
2. Jin GY, Lee JM, Lee YC, Han YM. Acute cerebral infarction after radiofrequency ablation of an atypical carcinoid pulmonary tumor. AJR Am J Roentgenol. 2004. 182:990–992.
3. Lee JM, Jin GY, Goldberg SN, Lee YC, Chung GH, Han YM, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology. 2004. 230:125–134.
4. Belfiore G, Moggio G, Tedeschi E, Greco M, Cioffi R, Cincotti F, et al. CT-guided radiofrequency ablation: a potential complementary therapy for patients with unresectable primary lung cancer-a preliminary report of 33 patients. AJR Am J Roentgenol. 2004. 183:1003–1011.
5. Steinke K, King J, Glenn DW, Morris DL. Percutaneous radiofrequency ablation of lung tumors with expandable needle electrodes: tips from preliminary experience. AJR Am J Roentgenol. 2004. 183:605–611.
6. Gadaleta C, Mattioli V, Colucci G, Cramarossa A, Lorusso V, Canniello E, et al. Radiofrequency ablation of 40 lung neoplasms: preliminary results. AJR Am J Roentgenol. 2004. 183:361–368.
7. Steinke K, Glenn D, King J, Clark W, Zhao J, Clingan P, et al. Percutaneous imaging-guided radiofrequency ablation in patients with colorectal pulmonary metastases: 1-year follow-up. Ann Surg Oncol. 2004. 11:207–212.
8. Lee JM, Han JK, Kim SH, Shin KS, Lee JY, Park HS, et al. Comparison of wet radiofrequency ablation with dry radiofrequency ablation and radiofrequency ablation using hypertonic saline preinjection: ex vivo bovine liver. Korean J Radiol. 2004. 5:258–265.
9. Lee JM, Kim SW, Li CA, Youk JH, Kim YK, Jin Z, et al. Saline-enhanced radiofrequency thermal ablation of the lung: a feasibility study in rabbits. Korean J Radiol. 2002. 3:245–253.
10. Lee JM, Han JK, Kim SH, Sohn KL, Lee KH, Ah SK, et al. A comparative experimental study of the in-vitro efficiency of hypertonic saline-enhanced hepatic bipolar and monopolar radiofrequency ablation. Korean J Radiol. 2003. 4:163–169.
11. Burdio F, Guemes A, Burdio JM, Castiella T, De Gregorio MA, Lozano R, et al. Hepatic lesion ablation with bipolar saline-enhanced radiofrequency in the audible spectrum. Acad Radiol. 1999. 6:680–686.
12. Burdio F, Burdio JM, Navarro A, Ros P, Guemes A, Sousa R, et al. Electric influence of NaCl concentration into the tissue in radiofrequency ablation. Radiology. 2004. 232:932.
13. Denys AL, De Baere T, Kuoch V, Dupas B, Chevallier P, Madoff DC, et al. Radio-frequency tissue ablation of the liver: in vivo and ex vivo experiments with four different systems. Eur Radiol. 2003. 13:2346–2352.
14. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.
15. Choi D, Lim HK, Kim MJ, Lee J, Kim SK, Kim EY, et al. Overlapping ablation using a coaxial radiofrequency electrode and multiple cannulae system: experimental study in ex-vivo bovine liver. Korean J Radiol. 2003. 4:117–123.
16. Lee JM, Youk JH, Kim YK, Han YM, Chung GH, Lee SY, et al. Radio-frequency thermal ablation with hypertonic saline solution injection of the lung: ex vivo and in vivo feasibility studies. Eur Radiol. 2003. 13:2540–2547.
17. Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology. 2001. 219:157–165.
18. Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G. A novel "cooled-wet" electrode for radiofrequency ablation. Eur Radiol. 2000. 10:852–854.
19. McGahan JP, Gu WZ, Brock JM, Tesluk H, Jones CD. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996. 3:418–422.
20. Curley SA, Davidson BS, Fleming RY, Izzo F, Stephens LC, Tinkey P, et al. Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endosc. 1997. 11:729–733.
21. Haemmerich D, Tungjitkusolmun S, Staelin ST, Lee FT Jr, Mahvi DM, Webster JG. Finite-element analysis of hepatic multiple probe radio-frequency ablation. IEEE Trans Biomed Eng. 2002. 49:836–842.
22. Haemmerich D, Staelin ST, Tungjitkusolmun S, Lee FT Jr, Mahvi DM, Webster JG. Hepatic bipolar radio-frequency ablation between separated multiprong electrodes. IEEE Trans Biomed Eng. 2001. 48:1145–1152.
23. Miao Y, Ni Y, Yu J, Zhang H, Baert A, Marchal G. An ex vivo study on radiofrequency tissue ablation: increased lesion size by using an "expandable-wet" electrode. Eur Radiol. 2001. 11:1841–1847.
24. Lobo SM, Afzal KS, Ahmed M, Kruskal JB, Lenkinski RE, Goldberg SN. Radiofrequency ablation: modeling the enhanced temperature response to adjuvant NaCl pretreatment. Radiology. 2004. 230:175–182.
25. Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996. 3:636–644.
26. Cosman ER, Nashold BS, Ovelman-Levitt J. Theoretical aspects of radiofrequency lesions in the dorsal root entry zone. Neurosurgery. 1984. 15:945–950.
27. Burdio F, Guemes A, Burdio JM, Navarro A, Sousa R, Castiella T, et al. Bipolar saline-enhanced electrode for radiofrequency ablation: results of experimental study of in vivo porcine liver. Radiology. 2003. 229:447–456.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download