Abstract
Fluorodeoxyglucose (FDG)-positron emission tomography (PET) is being used more and more to differentiate benign from malignant focal lesions and it has been shown to be more efficacious than conventional chest computed tomography (CT). However, FDG is not a cancer-specific agent, and false positive findings in benign diseases have been reported. Infectious diseases (mycobacterial, fungal, bacterial infection), sarcoidosis, radiation pneumonitis and post-operative surgical conditions have shown intense uptake on PET scan. On the other hand, tumors with low glycolytic activity such as adenomas, bronchioloalveolar carcinomas, carcinoid tumors, low grade lymphomas and small sized tumors have revealed false negative findings on PET scan. Furthermore, in diseases located near the physiologic uptake sites (heart, bladder, kidney, and liver), FDG-PET should be complemented with other imaging modalities to confirm results and to minimize false negative findings. Familiarity with these false positive and negative findings will help radiologists interpret PET scans more accurately and also will help to determine the significance of the findings. In this review, we illustrate false positive and negative findings of PET scan in a variety of diseases.
Differentiation between malignant and benign pulmonary nodules is a common problem encountered by radiologists which has provided the impetus to explore alternative imaging techniques. Accurate diagnosis can reduce unnecessary thoracotomies in patients with benign diseases (1). Metabolic imaging with 2-(18F)-fluoro-2-deoxy-D-glucose positron emission tomography (PET) is being used more and more to differentiate benign from malignant focal lesions and it has been shown to be more efficacious than conventional chest CT (2). It has a unique ability to differentiate benign from malignant nodules, and it offers a different approach to the diagnosis of chest diseases because it exploits fundamental biochemical differences between benign and malignant cells (2).
However, fluorodeoxyglucose (FDG) is not a cancer-specific agent, and false positive findings in benign diseases have been reported in active inflammation or infection, causing false-positive results (1, 2). In addition, the malignant tumors with low metabolic activity (3) or tumors smaller than 1.0 cm in diameter (1) often show false negative results. Furthermore, the accuracy of FDG-PET scans in detecting pulmonary metastases in patients with cancer has not yet been established.
Awareness of the conditions and the mechanisms by which false positive and negative results occur will help radiologists interpret PET scans more accurately and also will help to determine the significance of the findings. In this review, potential sources of false-positive and false-negative findings are illustrated in a variety of lung diseases.
Fluorodeoxyglucose is an analog of glucose and is used as a tracer of glucose metabolism. There are several sites of normal physiologic accumulation of FDG (Fig. 1). It accumulates in various normal organs which use glucose for metabolism, including the brain, muscles, salivary glands, myocardium, gastrointestinal tract, urinary tract, thyroid gland, and gonadal tissues. In additional, increased FDG uptake in brown adipose tissue in the neck has been reported as a false positive result in 2.3-4% of patients. Brown adipose tissue is responsible for cold-induced and diet-induced thermogenesis. Mitochondria in brown adipose tissue exclusively express the thermogenic protein, and FDG uptake in hypermetabolic brown fat can occur, as glucose transporters have been observed in brown adipose tissue (Fig. 2) (2).
The mechanism by which FDG uptake in tumor cells and other pathologic conditions occurs is due to an increased number of glucose transporter proteins and increased intracellular hexokinase and phosphofructokinase levels, which promote glycolysis. In the normal glycolytic pathway, FDG behaves similarly to D-glucose in its transport through the cell membrane and phosphorylation by hexokinase. Once FDG is phosphorylated, structural changes made by a hexose-phosphate bond prevent FDG from being catabolized or transported back into the extracellular space in substantial amounts. This process is called "metabolic trapping," and it makes increased uptake and accumulation of FDG occur within abnormally metabolizing tumor cells (2).
Abnormal areas of FDG accumulation are detected by comparing the uptake with background activity. In the lungs, focal abnormalities that have a greater uptake than the mediastinal blood pool on attenuation corrected images are highly suggestive of malignancy. FDG PET also provides quantitative data in the form of the standardized uptake value (SUV) or standardized uptake ratio (SUR) (2). The SUV is obtained by putting the circular region of interest over the portion of the lesion with the greatest accumulation of FDG (3). The mean SUV is obtained by dividing the activity (µCi/mL) in the region of interest (ROI) by the injected dose in µCi divided by patient weight in grams (3).
SUV = (average ROI activity [µCi /mL]) / (injected dose [µCi] per body weight [g])
Increased body fat elevates the SUV, therefore, correction with lean body mass (SUVLBM) is required to avoid erroneous comparisons that can result from changes in pre- and post-therapy body weight in the same patient (2). The maximum SUV is obtained by using the maximum activity in the ROI instead of the mean activity (3). Notwithstanding the controversial views, SUVs of 2.5 or greater have been used as a cutoff value indicative of malignancy (3). Gupta et al. (4) reported that FDG-PET is superior to CT, when considering overall sensitivity, specificity, and accuracy of PET for staging mediastinal lymph nodes was 96, 93, and 94%, compared to 68, 65, and 66% with CT.
Inflammatory cells such as neutrophil and activated macrophages at the site of inflammation or infection show increased FDG accumulation (5). Active granulomatous processes, other infectious conditions and active fibrotic lesions have also been reported to show increased FDG accumulation and cause false-positive PET scans for malignancy.
Tuberculoma is one of the most well-known diseases that show intense FDG uptake (Fig. 3A). Active granulomatous processes such as tuberculosis have been reported to accumulate FDG (1, 2). Tuberculoma typically appears as a fairly discrete nodule or mass (Fig. 3B) in which repeated extensions of infection have created a central caseous necrosis surrounded by a mantle of epithelioid cells and collagen with peripheral inflammatory cell infiltration (1).
Activated inflammatory cells have markedly increased glycolysis. The hexose monophosphate shunt is stimulated by phagocytosis, with increases of 20-30 times that of baseline values which is the cause of high FDG uptake (6). Tuberculous lymphadenopathy can be understood in the same manner as tuberculoma in the lung parenchyma (Fig. 4).
Elevated glucose levels can accelerate false positive results in inflammatory conditions. It has been suggested that inflammatory cells use more glucose under hyperglycemia than in euglycemia, and therefore, lesions containing such cells are more likely to be interpreted as malignant lesions under such conditions (5).
Sarcoidosis is a chronic multisystem disorder. It can be characterized in affected organs by an accumulation of T lymphocytes and mononuclear phagocytes, noncaseating epithelioid granulomas, and derangements of the normal tissue architecture. The etiology is unknown, but it is thought to be caused by exaggerated cellular immune responses.
Pathologically, the first manifestation of the disease is an accumulation of mononuclear inflammatory cells, mainly CD4+ T helper 1 lymphocytes and mononuclear phagocytes, in the affected organ. This inflammatory process is followed by the formation of granulomas, aggregates of macrophages and their progeny, epithelioid cells, and multinucleated giant cells (7). Active granulomatous conditions with aggregation of inflammatory cells in sarcoidosis results in accumulation of FDG (1-3, 8), and it has been suggested that intensity of FDG uptake may reflect disease activity (9) (Fig. 5).
Cryptococcosis is an infection caused by the yeast-like fungus Cryptococcus neoformans. This fungus reproduces by budding and forms round, yeast-like cells. Infection occurs by inhaling the fungus into the lungs. Pulmonary lesions are characterized by intense granulomatous inflammation (10). There are some reports which have showed high FDG uptake causing false positive results in cryptococcosis (8) (Fig. 6).
Paragonimiasis is a food-borne parasitic disease common in south Asia, particularly in Japan, Korea and parts of China (11). Pulmonary paragonimiasis is a disease caused by a lung flake. Once ingested by humans, by eating infected crabs or crayfish, the larvae excyst in the small intestine, penetrate the intestinal wall, and enter the peritoneal cavity. They then penetrate the diaphragm and pleura and enter the lungs. The major target organs for the larvae are the lungs followed by the brain (12).
During the active phase of paragonimiasis, lung tissue surrounding the parasitic cysts may contain evidence of pneumonia, bronchitis, bronchiectasis, and fibrosis (12) (Fig. 7A). Watanabe et al. (11) reported one case of paragonimiasis mimicking lung cancer which showed high FDG uptake (SUV 4.7 at one hour post-injection, and elevation of SUV to 6.2 at two hours). Although the exact causes of FDG accumulation have not yet been proven, we can expect that inflammatory cells including eosinophlic infiltration, active inflammatory responses and viable worms cause high FDG uptake (Fig. 7B).
In variable inflammatory conditions including abscesses (Fig. 8), glycolytic metabolism is elevated in the region of leukocytic infiltration associated with inflammatory processes, and consequently FDG uptake is elevated (2). Pneumocystis infections can also cause high FDG-uptake (Fig. 9). These conditions have not been well documented to date. However, in one report (13), patients with fever of unknown origin presented with high FDG uptake in PET imaging, finally proved to be infected by pneumocystis carinii pneumoniae. Inflammatory cells increase the expression of glucose transporters when they are activated, and multiple cytokines and growth factors can facilitate glucose transport without actually increasing the number of glucose transporters (5).
Inflammatory processes in radiation fibrosis is caused by infiltration of leukocytes and macrophages and abnormal proliferation of type II pneumocytes, radiation-induced production of local cytokines such as platelet-derived growth factor, tumor necrosis factor, and transforming growth factor β in the radiation field are based on these inflammatory morphologic changes. Consequently, increased FDG uptake in the affected region would be expected (Fig. 10) (14).
Pneumoconiosis is a tissue reaction to the presence of an accumulation of dust in the lungs. One clinicopathologic form of this reaction is fibrosis, while the other form consists of aggregates of particle-laden macrophages with minimal or no accompanying fibrosis, a reaction that is typically seen with inert dusts such as iron, tin, and barium (15) (Fig. 11). FDG-PET studies have revealed increased uptake in pneumoconiosis and progressive massive fibrosis. Some of this uptake is perhaps related to the presence of inflammatory cells such as macrophages, as well as fibroblasts (5).
Sclerosing hemangioma is widely believed to arise from type II pneumocyte and bronchial epithelial lining. It is a benign lesion with a good prognosis. However, in some cases it shows local invasion, multiplicity and lymph node metastasis. The CT findings of scelrosing hemangiomas are well defined, round or oval shaped, enhanced mass with or without punctuate calcification. There has been only one case report of FDG-PET findings of sclerosing hemangioma, and in this report it shows intermediate FDG uptake (SUV= 3.0) (16) (Fig. 12).
Even within tumors, FDG uptake is not completely confined to the tumor cells themselves. The newly formed granulation tissue around the tumor and the macrophages infiltrating the marginal areas surrounding the necrotic area of the tumor show a high uptake of FDG. Some authors have suggested that about 24% of the FDG concentration in a tumor mass is derived from non-tumor tissue (17).
Tumors with low activity are well-known major causes of false negative findings. This can be easily understood considering the FDG PET is metabolic imaging using the activity of lesion.
Another major cause of false negative findings for malignancy is tumor size. It is likely a result of a loss in measured activity as the nodule size diminishes due to the roughly 1-cm resolution of the PET systems frequently used (partial volume effect) (2).
Bronchioloalveolar carcinomas appear as areas of ground-glass opacity, nodules, masses, or areas of ground-glass opacity plus consolidation, on high-resolution CT scans. On pathologic examination, bronchioloalveolar carcinomas are well differentiated, having moderate degrees of nuclear atypism, mild degrees of mitotic figure, desmoplasia, and necrosis. These mild degrees of atypism, mitosis, and desmoplasia may be the causes of lower peak SUVs than those of other lung carcinomas. Several reports (18) have revealed lower FDG uptake in BAC than adenocarcinomas in the lungs. Thus, bronchioloalveolar carcinomas can be potential causes of false negative findings of malignancy on FDG PET scans (Fig. 13). Furthermore, mucinous bronchioloalveolar cell carcinomas, which often contain abundant mucin, exhibit significantly lower peak SUVs compared with those of squamous cell carcinomas, adenocarcinomas, and other cell types. In such cases, not only histologic grade, but also the amount of mucin component in the tumor (Fig. 14) is closely related with FDG-uptake (18).
When lesions are smaller than 1 cm, these lesions may not show high FDG uptake in the lungs or mediastinum. This is the result of the 1-cm resolution of PET systems frequently used (partial volume effect) (2). Spatial resolution limitations of FDG PET have been shown to be the causative factor of false negative PET scans (Fig. 15).
Metastasis of tumors with a mucinous component can result in low FDG uptake (18). The relative cellularity of tumors is important in the detection of disease using FDG PET. Consequently, low cellularity in these tumors caused by the presence of mucin results in lower FDG uptake (19). For example, lung metastasis of a mucinous carcinoma of the breast (Fig. 16) may not show high FDG uptake. Other metastatic tumors such as mucinous adenocarcinomas of gastrointestinal origin can also show false negative findings in PET scans. Also, a metastatic mass from a renal cell carcinoma (20) (Fig. 17), and some invasive ductal and lobular breast carcinomas (21) are well reported to result in false negative findings.
In several previous reports, it has been well documented that FDG uptake usually decreases after chemotherapy, and this correlates with therapeutic response. Decreased FDG uptake after irradiation is mainly due to the reduced number of metabolically active tumor cells. However, a decrease of FDG PET does not always predict a good response because FDG can differentiate metabolically active cells from dead cells, but cannot differentiate biologically viable from metabolically active cells (22) (Fig. 18).
Carcinoid tumors arise from the dispersed neuroendocrine system, and are classified as typical and atypical, depending on the degree of cellular atypia. In a previous report, the SUV in a carcinoid tumor was 1.0-2.3, which is the result of low metabolic activity. The reason for the low metabolic activity in the carcinoid tumor is unknown (23).
The biodistribution of FDG can be affected by blood glucose levels. In situations of high blood glucose levels, circulating blood glucose uptake in pathologic lesions increase and this prevents FDG uptake because of the competitive reaction (5). Therefore, a large amount of glucose in the blood is taken up in the tumor tissue which interferes FDG uptake in the tumor, resulting in a false negative finding. Specifically, in type II diabetic patients insulin may be used to manipulate glucose levels. However, there is still no agreement as to adjusting glucose levels in diabetic patients (2).
Metabolic imaging with FDG-PET is beginning to play important role in the management of oncologic diseases. In addition, it plays a complementary role in accurate overall evaluation of malignant diseases. However, false positive FDG uptake or false negative PET scans are frequently encountered. Proper interpretation and accurate characterization of an abnormality can be accomplished only if one is aware of possible false positive and negative conditions.
Figures and Tables
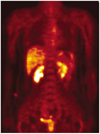 | Fig. 1Normal distribution of FDG.
Coronal FDG-PET image shows physiologic uptake in the liver, kidneys, intestine, and urinary bladder. Also note the minimal uptake in the mediastinum, and bone marrow.
|
 | Fig. 3Tuberculoma in a 53-year-old female.
A. Contrast-enhanced CT scan shows a round mass in the left lower lobe.
B. Axial FDG-PET image shows intense uptake (arrow) in the left upper lobe suggesting a malignant condition with a maximum standardized uptake value of 4.3. The pathologic examination reveals tuberculoma. Another lesion showing high FDG uptake (arrowhead) is a pulmonary artery.
|
 | Fig. 4Tuberculous lymphadenitis in a 56-year-old male.
A. Axial contrast-enhanced CT scan shows right hilar lymph node enlargement (arrow).
B. Coronal FDG-PET scan shows high uptake in the same area (arrow).
|
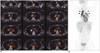 | Fig. 5Sarcoidosis in a 32-year-old male.
A. Axial PET-CT scans show bilateral paratracheal and hilar lymph node enlargement with high FDG uptake.
B. Coronal section of PET shows high FDG uptake with rhamda shape (arrowheads) which is highly suggestive of sarcoidosis. A mediastinoscopic biopsy confirms sarcoidosis.
|
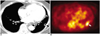 | Fig. 6Cryptococcosis in a 68-year-old female.
A. Contrast-enhanced CT scan shows a cavitary nodule (arrow) in the left lower lobe.
B. Transverse section of a whole body PET image shows increased uptake (arrow) in the left lower lobe and a standardized uptake value of 2.6. The lesion is a round mass-like lesion unlike the CT findings due to respiration artifact.
|
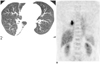 | Fig. 7Paragonimiasis in a 68-year-old male.
A. In the lung window setting, axial transverse CT scan shows linear, wedge shaped consolidation and small centrilobular nodules. Radiologic diagnosis is atypical tuberculosis.
B. Coronal section of FDG-PET image shows intense uptake in the right lower lung zone. Bronchoscopic washing and sputum cytology reveals many parasitic eggs of paragonimus species.
|
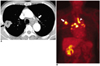 | Fig. 8Lung abscess in a 72-year-old male.
A. Axial contrast enhanced CT scans show a consolidation with air density in the right upper lobe (black arrow). Multiple conglomerated mediastinal lymph node enlargements are also noted (white arrow).
B. Coronal FDG-PET image shows high uptake (arrows) in the right upper lobe. High uptake in the left hilar lymph node (arrowhead) is also noted. Malignancy is suspected in PET scan. Percutaneous needle biopsy reveals a lung abscess.
|
 | Fig. 9Pneumocystis carinii infection in a 61-year-old female.
A. In the lung window setting of chest CT, multifocal ground-glass opacities are noted. The patient had been diagnosed with systemic lupus erythematosus, and cytoxan and steroid pulse therapy was performed.
B. PET-CT shows multifocal hypermetabolic lesions with an standardized uptake value of between 4.0-5.8 in both lung parenchymas.
|
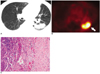 | Fig. 10Radiation fibrosis in a 61-year-old male by radiation therapy due to lung cancer.
A. Axial CT scan shows consolidation in the left lower lobe, suggesting lung cancer recurrence.
B. Axial PET image shows increased uptake in the left lower lobe (arrow) which was mistaken for lung cancer recurrence.
C. Microscopic image reveals severe fibrosis with inflammatory cell accumulation.
|
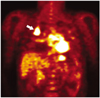 | Fig. 11Progressive massive fibrosis in a 58-year-old male.
Coronal FDG-PET image shows increased uptake in right upper lobe (arrow) and a mean standardized uptake value of 6.4.
|
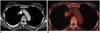 | Fig. 12Sclerosing hemangioma in a 43-year-old female.
A. Axial contrast-enhanced CT shows a round well demarcated, highly enhancing mass in the right lateral side of the superior vena cava in the right upper lobe.
B. FDG-PET fusion CT image shows increased uptake in the lesion with a maximum standardized uptake value of 3.4.
|
 | Fig. 13Bronchioloalveolar carcinoma in a 75-year-old male.
A. Contrast-enhanced CT shows a cavitary lesion in the left lower lobe (arrow).
B. Transverse FDG-PET image of the transverse scan shows subtle uptake (arrow) in the left lower lobe with a maximum standardized uptake value of 1.7.
|
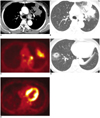 | Fig. 14Mucinous bronchioloalveolar carcinoma in a 61-year-old female.
A, B. Axial chest CT images show a low attenuating mass in the left upper lobe.
C. FDG-PET also shows a hypermetabolic lesion with a maximum standardized uptake value of 3.8 in the left upper lobe.
D. Another area of ground-glass opacity is noted in the right lower lobe.
E. FDG-PET shows no abnormal uptake in this area. Both the left upper and right lower lobe lesions were bronchioloalveolar carcinomas, mucinous type. In the same patient, FDG uptake for each of the lung lesions was different, and the amount mucin in mass may have been the major cause of this difference.
|
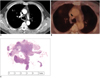 | Fig. 15Small malignant mediastinal lymph node in a 56-year-old male.
A. Axial contrast-enhanced CT shows a nodular lesion in the right upper lobe with a lymph node of less than 1 cm in the right paratracheal area (arrow).
B. FDG-PET fusion CT shows no increased uptake due to the small size of the lesion (arrow).
C. Pathology reveals a 0.6 cm lymph node with metastatic tumor cells.
|
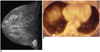 | Fig. 16Metastatic lung nodule from mucinous carcinoma of the left breast.
A. Gadolinium-enhanded MR shows a well enhancing lobulating mass.
B. FDG-PET fusion CT shows a nodular lesion in the right lower lung without increased FDG uptake (arrow).
|
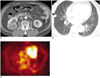 | Fig. 17Metastasis from renal cell carcinoma in an 83-year-old male.
A. Axial contrast-enhanced CT scans shows a mass lesion in the right kidney suggesting renal cell carcinoma.
B. Axial CT scan of lung window setting shows a lung nodule in the right middle lobe (arrow).
C. A selected transverse section of whole body-PET image shows no demonstrable uptake in the right lung.
|
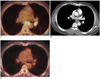 | Fig. 18Post-chemotherapy related decreased lymph node uptake in a 57-year-old male lung cancer patient.
A. Pre-chemotherapy FDG-PET fusion CT shows increased FDG uptake in the subcarinal lymph node with a maximum standardized uptake value of 9.4.
B. After two months of chemotherapy, the residual lesion in the subcarinal area is noted.
C. FDG-PET fusion CT shows no abnormal FDG uptake.
|
References
1. Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology. 2000. 216:117–121.
2. Kostakoglu L, Agress H, Goldsmith SJ. Clinical role of FDG PET in evalutaion of cancer patients. RadioGraphics. 2003. 23:315–340.
3. Trukington TG, Coleman RE. Clinical oncologic PET: An Introduction. Semin Roentgenol. 2002. 37:102–109.
4. Gupta NC, Graeber GM, Bishop HA. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (< 1 cm), intermediate (1 to 3 cm), and large (> 3 cm) lymph node lesions. Chest. 2000. 117:773–778.
5. Alavi A, Gupta N, Alberini JL, Hickeson M, Adam LE, Bhargava P, et al. Positron emission tomography Imaging in nonmalignant thoracic disorders. Semin Nucl Med. 2002. 32:293–321.
6. Amerein PC, Larson SM, Wagner HN Jr. An automated system for measurement of leukocyte metabolism. J Nucl Med. 1974. 15:352–355.
7. Crystal RG . Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Disorders of the immune system, connective tissue, and joints, sarcoidosis. Harrison's principle of internal medicine. 2001. 15th ed. NewYork: McGraw-Hill;1969–1970.
8. Bakheet SM, Powe J. Benign causes of 18-FDG uptake on whole body imaging. Semin Nucl Med. 1998. 28:352–358.
9. Brudin LH, Valind SO, Rhodes CG, Pantin CF, Sweatman M, Jones T, et al. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994. 21:297–305.
10. Bennett JE. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Cryptococcosis. H arrison's principle of internal medicine. 2001. 15th ed. NewYork: McGraw-Hill;1174–1175.
11. Watanabe S, Nakamura Y, Kariatsumari K, Nagata T, Sakata R, Zinnouchi S, et al. Pulmonary paragonimiasis mimicking lung cancer on FDG-PET imaging. Anticancer Res. 2003. 23:3437–3440.
12. Im JG, Kong Y, Shin YM, Yang SO, Song JG, Han MC, et al. Pulmonary paragonimiasis: clinical and experimental studies. RadioGraphics. 1993. 13:575–586.
13. Lorenzen J, Buchert R, Bleckmann C, Munchow N, Bohuslavizki KH. A search for the focus in patients with fever of unknown origin: is positron-emission tomography with F-18-fluorodeoxyglucose helpful? Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 1999. 171:49–53.
14. Lin P, Chu J, Pocock N. Fluorine-18 FDG dual-head gamma camera coincidence imaging of radiation pneumonitis. Clin Nucl Med. 2000. 25:866–869.
15. Speizer FE. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Environmental lung diseases. Principle of internal medicine. 2001. 15th ed. NewYork: McGraw-Hill;1470–1471.
16. Hara M, Iida A, Tohyama J, Miura N, Shiraki N, Itoh M, et al. FDG-PET findings in sclerosing hemangioma of the lung: a case report. Radiat Med. 2001. 19:215–218.
17. Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992. 33:1972–1980.
18. Kim B, Kim Y, Lee K, Yoon SB, Cheon EM, Kwon OJ, et al. Localized form of bronchioloalveolar carcinoma: FDG PET findings. AJR Am J Roentgenol. 1998. 170:935–939.
19. Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000. 174:1005–1008.
20. Montravers F, Grahek D, Kerrou K, Younsi N, Doublet JD, Gattegno B, et al. Evaluation of FDG uptake by renal malignancies (primary tumor or metastases) using a coincidence detection gamma camera. J Nucl Med. 2000. 41:78–84.
21. Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxtglucose: use and limitations. J Clin Oncol. 2000. 18:3495–3502.
22. Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002. 35:179–187.
23. Erasmus JJ, McAdams HP, Patz EF Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol. 2004. 182:559–567.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download