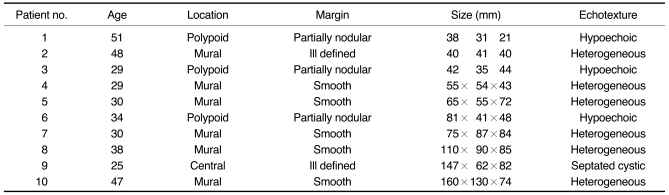
RESULTS
The preoperative sonographic diagnoses were stromal sarcoma (n = 4,
Figs. 1,
2), uterine malignancy (n = 1,
Fig. 3), leiomyoma (n = 4), and adenomyosis (n = 1,
Fig. 4). On the sonographic images, the lesions were observed as being in the uterine wall in six cases (
Fig. 1), as a polypoid mass protruding into the endometrial cavity from the myometrium in three cases (
Fig. 2), and as a central cavity mass in the remaining one case (
Fig. 3,
Table 1). The masses were ill defined from the endometrium in six cases and well defined from the endometrium in four cases (patients 4, 5, 7, and 8). The lesion margins were smooth (n = 5), ill defined (n = 2) and smooth with partially nodular extensions (n = 3,
Table 1). The maximal length of the masses ranged from 38 mm to 160 mm with a mean mass length of 83.5 mm (
Table 1). A single lesion was noted in eight cases and multiple masses were noted in two (patients 4 and 6). The echo-textures of the lesions were a homogeneous hypoechoic mass (n = 3), a heterogeneously intermediate echoic mass (n = 5), diffuse myometrial thickening with heterogeneous echogenicity (n = 1,
Fig. 4), and a septated cystic mass (n = 1,
Table 1).
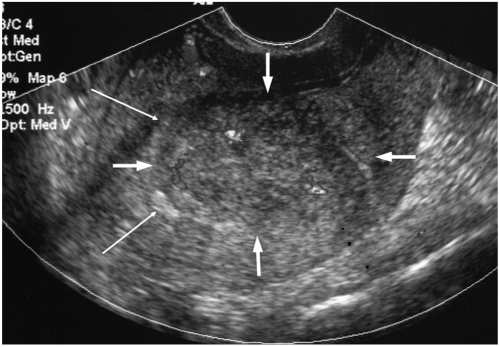 | Fig. 1
Sonographic findings of endometrial stromal sarcoma that presented as a mural mass in patient 2.
A heterogeneously echoic mass with an ill defined margin is noted in the uterine wall (thick arrows). The endometrium is displaced by the mass (thin arrows). Color Doppler sonography demonstrates a focally dispersed vascularity within the mass.
Pathology revealed a subendometrial endometrial stromal sarcoma invading more than half of the myometrial thickness (not shown).

|
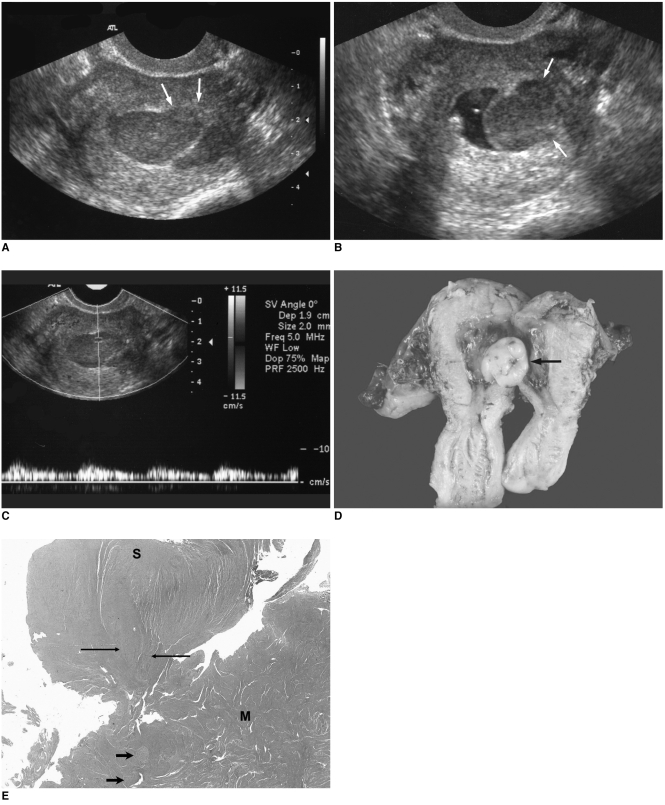 | Fig. 2Sonographic findings of endometrial stromal sarcoma, which presented as a polypoid mass protruding into the endometrial cavity from the myometrium in patient 1. 
|
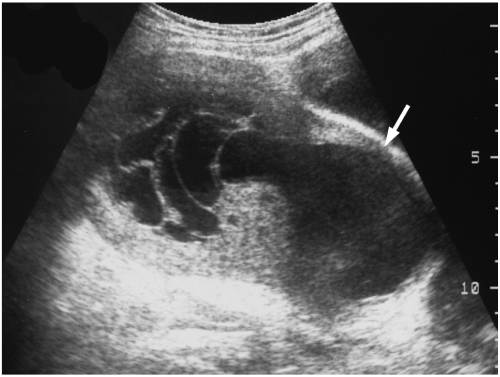 | Fig. 3
Endometrial stromal sarcoma that presented as a huge central cavity mass.
An ill defined, septated cystic mass with a solid portion is noted in the uterine central cavity in patient 9. The endometrium is obliterated by the mass and myometrial thinning is noted (arrow).
Pathology revealed a low grade endometrial stromal sarcoma invading the endometrium and almost the full thickness of the anterior myometrium and cystic degeneration of the tumor (not shown).

|
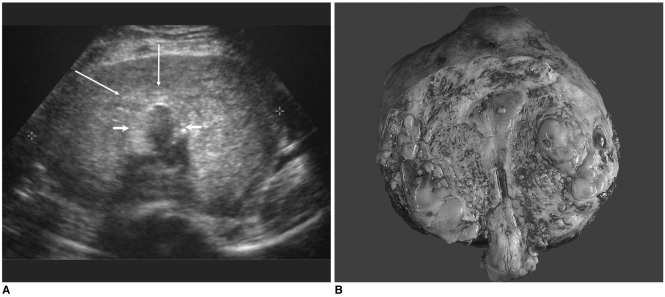 | Fig. 4Endometrial stromal sarcoma that presented with diffuse myometrial thickening. 
|
The color Doppler studies showed focally dispersed irregular short vasculature in five cases, and pulsed Doppler studies showed pulsatile vascularity with a measured resistance index (RI) of 0.33 to 0.39 and a pulsatility index (PI) of 0.35 to 0.49 in three cases (
Fig. 2C).
The pathology results revealed myometrial invasion in all cases and endometrial invasion in three cases (patients 1, 6, 9). Polypoid masses with myometrial invasion were revealed in two of the three cases in which a polypoid mass protruding into the endometrial cavity was suggested on sonography (
Fig. 2). Submucosal mass without endometrial invasion was verified in the remaining one case. An ill defined central cavity mass with endometrial and myometrial invasion was noted in the case that presented as a central cavity mass on sonography. The lesions were confined to the myometrium in the cases that presented as mural masses. Extension beyond the uterine serosa and invasion of the adjacent structures were noted in two cases: the ovary and pelvic wall for patient 10, and the mesentery and descending colon for patient 6.
Go to :

DISCUSSION
Endometrial stromal sarcoma is a malignant neoplasm that shows endometrial stromal differentiation. ESS usually displays indolent clinical behavior, but it has a propensity for recurrence and metastasis (
1,
2). Its usual clinical impression is of a uterine leiomyoma presenting with unusual vaginal bleeding. The tumor is nearly always begins in the endometrium or myometrium. ESS is differentiated from an endometrial stromal nodule (ESN) by infiltrative myometrial invasion and its characteristic tongue-like growth (
6). The differential diagnosis between ESS and ESN is not possible with using curettage specimens (
7). These tumors have generally been divided into low- and high-grade ESSs on the basis of whether the maximal mitotic rate was less or greater than 10 mitoses per 10 high-power fields (
8). ESS currently includes only the cases that were classified as low grade ESSs in the previous studies. Those tumors that were classified as high grade ESS in previous studies were categorized as poorly differentiated endometrial sarcoma because they behaved aggressively and the pathology results revealed no specific evidence of an endometrial origin in them (
2,
8). The gross pathologic changes in the uterus are a myometrial mass, diffuse thickening of the uterine wall and a polypoid mass protruding into the endometrial cavity (
2,
6).
The sonographic findings of ESS have been described in only a few cases. The reported sonographic findings are nonspecific and variable, i.e., a protruding mass into the uterine cavity from the myometrium or poorly defined uterine masses with solid components or mixed cystic and solid components (
3,
4). The malignant potential was suggested by color Doppler sonography showing irregular dispersed vessels with low RI values within the mass (
3-
5). The magnetic resonance (MR) imaging findings of ESS have been reported to vary from a polypoid endometrial mass to diffuse myometrial thickening (
9-
11). The authors of these previous studies reported that MR imaging can provide a diagnostic clue by demonstrating an endometrial origin and myometrial invasion, but no truly specific diagnostic finding was identified (
9-
11).
In the present study, the sonographic findings were variable and four patterns of appearance were suggested; a mural mass or masses (n = 5, patients 2, 4, 5, 7, and 8), a polypoid mass protruding into the endometrial cavity from the myometrium (n = 3, patients 1, 3, and 6), a huge central cavity mass (n = 1, patient 9), and diffuse myometrial thickening (n = 1, patient 10).
Leiomyoma is a major consideration for the sonographic differential diagnosis of ESS because the majority of leimyomas occur in the premenopausal women. We made a preoperative diagnosis of leiomyoma based on the sonographic findings in four patients; intramural leiomyomas (n = 3) and a submucosal leiomyoma (n = 1). Differentiation between the intramural mass type of ESS and leiomyoma is difficult, but the present study identified some differences between ESS and leiomyoma. The margins of the ESS masses are less well defined and lack the hypoechoic peripheral rim that is frequently observed in leiomyoma. Moreover, the ESS masses are more heterogeneously echogenic whereas leiomyomas tend to be hypoechoic. In addition, the whirling heterogeneity pattern frequently visualized in leiomyomas is lacking in ESS.
Submucosal leiomyoma was considered in the differential diagnosis for the polypoid mass type ESS, but an ill defined margin against the endometrium and a nodular extension to the myometrium suggested the possibility of malignancy of an endometrial origin.
In the huge central cavity type mass, the mass margins were ill defined and the endometria were nearly obliterated. The echogenicity of this type of mass was septated cystic. The possibility of a uterine sarcoma was suggested. The pathology results revealed a polypoid mass located in the endometrial cavity with myometrial invasion and focal cystic change of the tumor. Endometrial thickening is associated with this type of tumor. A septated cystic appearance of ESS is rare; only a single such case has been reported on (
12). Those authors reported that the cyst wall was surrounded by cells having an endometrial stromal differentiation and there were cysts that contained clear fluid (
12). The considerations of the differential diagnosis for the huge central cavity mass include malignant mixed mesodermal tumor (MMMT) and endometrial carcinoma. These tumors tend to occur later in life and the most common presenting complaint is postmenopausal vaginal bleeding, whereas ESS occurs in premenauposal women (
1). MMMT shows aggressive clinical behavior and a heterogeneous sonographic appearance (
13). Most endometrial carcinomas present as endometrial thickening with increased echogenicity on sonography (
14). However, the differential diagnosis of these tumors is not possible with only performing sonography and additional studies are required to obtain the cytologic diagnosis.
In the type of mass with diffuse myometrial thickening, the myometrium was thickened and it showed heterogeneous echogenicity, and the impression was that of adenomyosis. The differential diagnosis between this type of ESS and adenomyosis is almost impossible, but a retrospective review revealed a more nodular, coarser appearance of the myometrium in ESS than in adenomyosis. In our case, the pathology results revealed numerous tiny nodules of ESS invading almost the full myometrial and uterine serosal thickness (
Fig. 4).
In the present study, although the color Doppler and pulsed Doppler sonographic findings were nonspecific, the malignant potential was suggested by an irregularly dispersed vascularity and a low mass impedance flow.
To the best of our knowledge, this report describes the sonographic findings of uterine ESS in the largest number of patients reported on to date. Although the sonographic findings of ESS are variable and nonspecific, some sonographic findings were found to suggest a diagnosis of ESS. Findings of a polypoid mass with nodular extension into the myometrium and an ill defined border against the endometrium, an intramural mass with an ill defined margin and heterogeneous echogenicity, the absence of the whirling pattern or the hypoechoic rim of leiomyoma or a large central cavity mass with an ill defined border, all of these suggest the possibility of ESS in the setting of a relatively young woman with abnormal uterine bleeding. With the suggestion of ESS on sonography, additional study is required to obtain a definite pathologic diagnosis.
In conclusion, ESS presents with four patterns of sonographic appearance; a polypoid mass with nodular extension into the myometrium, an intramural mass with an ill-defined margin and heterogeneous echogenicity, an ill-defined large central cavity mass and last, diffuse myometrial thickening. The location of the mass as related to the endometrium, a nodular myometrial extension and the appearance of an intramural mass with an ill defined border and heterogeneous echogenicity in premenopausal women could be the findings that suggest the diagnosis of uterine ESS.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download