Abstract
Objective
We wanted to assess the usefulness of four-dimensional (4D) ultrasonography (US), i.e., real-time three-dimensional US, as an adjunct for performing various US-guided interventional procedures in superficial lesions.
Materials and Methods
Thirty-three patients were referred for US-guided interventional procedures for superficial lesions, including core biopsy in 19, fine-needle aspiration in eight, therapeutic drug injection in four and needle puncture in two. The procedures were performed under 4D US guidance. We reviewed the pathologic/cytologic results of the core biopsies or needle aspirations, and also the outcomes of drug injection or needle puncture.
Results
For all the patients who underwent 4D US-guided core biopsy, the specimens were adequate for making the pathological diagnosis, and specimens were successfully obtained for those patients who underwent 4D US-guided aspiration. The patients treated with 4D US-guided therapeutic drug injection or needle puncture had a good response. No major procedure-related complications occurred. The procedural times were similar to those procedural times with using two-dimensional US.
Ultrasonography (US)-guided interventional procedures are widely used for both diagnostic and therapeutic purposes to treat a variety of disease entities (1, 2). Examples of such procedures include core biopsy, fine-needle aspiration and other various treatments. Moreover, the refinement of ultrasound machines and the interventional techniques has expanded their clinical applications. For example, the evolution of high-resolution US has made US-guided interventional procedures more suitable for superficial lesions than they were previously. By using a conventional ultrasound scanner, these procedures are performed by using two-dimensional (2D) US with real-time visualization of the investigated structures. The location of the biopsy needle and the anatomic or pathologic structures are judged on the real-time single-planar images that are obtained along the axis of the US transducer.
Three-dimensional (3D) US is an advanced US technique in which a large number of parallel or nearly parallel 2D US images are acquired with a high frame rate and then they are assembled into a single volume dataset. The information in the volume dataset undergoes instant image reconstruction and this is displayed by using various rendering techniques, including surface rendering, volume rendering and multiplanar reformatting (3, 4). The rapid data acquisition and display of the imaging by the contemporary 3D US scanners makes it possible to use four-dimensional (4D) US imaging, i.e., real-time 3D US display, in clinical applications (3). 4D US allows the physician to monitor the position of the needle in a real-time 3D display during the interventional procedure. There have been several articles that have described the clinical application of static 3D US in various interventional procedures (5-11), but the reports regarding the application of 4D US-guided procedures are rather limited (12). Therefore, the objective of this study was to evaluate the application of 4D US as an adjunct to 2D US for performing guided interventional procedures in superficial lesions.
We enrolled 33 consecutive patients who underwent 4D US-guided interventional procedures for superficial lesion during a 2-year period (from September 2002 to October 2004). They included 17 men and 16 women with an age range of 13-82 years (mean age: 48 years). Nineteen patients with initial clinical impression of neoplasm had been referred for US-guided core biopsy (range of tumor size: 2.2 to 9.2 cm, mean size: 4.7 cm). Eight patients with previous imaging diagnosis of a small nodule (range of nodule size: 0.5 to 2.9 cm in long axis, mean size: 1.3 cm) had been referred for needle aspiration (in four of these eight patients, the nodule is impalpable). Three patients had been referred for needle aspiration to prove urate crystal deposition. One patient had been referred for needle aspiration of a suspicious subdeltoid ganglion. Four patients had been referred for therapeutic drug injection (range of targeted lesion size: 2 to 7.3 cm, mean size: 3.8 cm), including corticosteroid injection to treat bursitis or fasciitis in three patients and LN immune therapy in one patient. Two patients had been referred for needle puncture for management of rotator cuff calcific tendonitis (size of calcified plaque: 12 mm). All the patients provided a written informed consent before their procedures.
An automated biopsy needle (Temno, Allegiance, McGraw Park, IL) was used for US-guided core biopsy. A syringe with a 22-gauge needle was used for the fine needle aspirations, the therapeutic drug injections or needle punctures.
The procedures were performed with using a 3D US machine (Voluson 730 Expert; GE Medical Systems/Kretztechnik, Zipf, Austria), that was equipped with a 6-MHz to 12-MHz mechanically driven curved-array transducer. The machine provided a dual-screen format that simultaneously displayed the real-time 2D axial images and the 4D volume-rendered images. The latter images displayed the coronal reformatting plane in the selected volume box with a 50%/50% mix of the surface mode and the transparent maximal mode. One radiologist (H-J.C.) performed the procedures by using a freehand technique with one hand holding the transducer and the other hand handling the needle. A technician adjusted the parameters on the panel of the US machine to achieve the best imaging quality during the procedures. At the beginning of the procedure, the 2D axial images were used for guidance, starting from the needle penetration of the skin to the tip of the needle approached the margin of the targeted lesion. This stage of the procedure was the same as was done in our previous study (13). We then activated the 4D US volume acquisition before we continued with the procedure. The location and volume acquisition angle of the volume box for the 4D volume-rendered image were optimally selected according to the location and the field of the needle path for each targeted lesion. 4D US volume acquisition was used to guide the needle tip to penetrate the lesions. The relationships of the needle tips within the lesions were monitored by using the realtime 2D axial and 4D volume-rendered images, which were simultaneously displayed on a dual-screen format. For the biopsy cases, three to six specimens were taken based on the continuity of the specimens. Each specimen was put in small formalin bottles and then they were sent to the department of pathology. In the aspiration cases, we used a 10 cc syringe connected with a 22# needle that was repeatedly passed through the nodule three to five times. During aspiration, the syringe was kept at 5 cc negative pressure and the needle was focused on the lymph node cortex or the center of the tiny nodule. For drug injection, 10 mg kenakort mixed with 1 cc distilled water and 1 cc 2% xylocaine was applied to manage the inflammatory process. For performing immune therapy with dendritic cell injection (a culture from the patient's immune system), the drug was injected into the cortex of the lymph node.
We reviewed the pathologic and cytologic results of the specimens that were obtained during core biopsy or needle aspiration. The therapeutic outcomes were evaluated for the patients who received therapeutic drug injection or needle puncture. After biopsy, the biopsy site was compressed by a sand bag for at least two hours. For the patients who received aspiration or injection, hand compression for about half to one hour was adequate. Every patient was followed up each day for at least three days after the procedure by an appointment if the patient was in the ward and a phone call was used if the patient was referred to the out-patient department.
Table 1 summarizes the results of the 4D US-guided interventional procedures for the 33 patients.
Nineteen patients received 4D US-guided core biopsy of superficial lesions (Fig. 1). All the procedures were performed successfully. The entire procedure from needle insertion to the finish of the procedure took about 20 minutes for the core biopsies, 10 minutes for the aspirations and five minutes for the drug injections and repeated punctures were usually necessary. There was no significant prolonged time as compared with our previous study (13). The pathologists were able to make the pathological diagnosis on the basis of the specimen obtained during 4D US-guided core biopsy in 18 (95%) patients. Seven patients had a pathological diagnosis of a nontumoral lesion (either an inflammatory process or fibrosis of the musculoskeletal system). Five of the seven patients had no evidence of tumor growth at the 1-year clinical follow-up after the procedure. The remaining two patients, who had limited clinical follow-up after biopsy, were identified as having osteomyelitis (one patient) and tuberculosis (one patient). Eleven patients who received 4D US-guided core biopsy had evidence of tumor growth on the pathologic examinations. The last patient, who presented with a history of polyarthralgia, developed a mass lesion in the left sternoclavicular joint. This patient received 4D US-guided biopsy of the lesion and the pathologic specimen showed nonspecific chondroid tissue. Repeat biopsy under 2D US guidance was performed two months later because of an inconclusive pathologic result, and the pathological findings from the second biopsy were similar to the first biopsy with no evidence of neoplasm. The lesion did not progress during 18-month follow-up; it was thought to be arthropathy that involved the left sternoclavicular joint.
Eight patients received 4D US-guided needle aspiration of their superficial lesions (Figs. 2, 3). All the procedures were performed successfully. Three of the patients had a clinical presentation of a gout attack. Monosodium urate crystals were identified in the aspirated specimens upon microscopic examination, which confirmed the clinical suspicion of gout. One patient with nasopharyngeal carcinoma presented with an enlarged lymph node (1.9 cm) in the right periauricular region, and this patient was diagnosed as having metastatic nasopharyngeal carcinoma after 4D US-guided aspiration. Two patients with enlarged lymph nodes in the neck (0.8 cm) or the inguinal region (2.9 cm) underwent 4D US-guided needle aspiration, and this showed no evidence of malignancy. These two patients had no evidence of progressive change in their nodal status at the 1-year clinical follow-up. One patient with a hypoechoic nodule in the right thyroid (1.8 cm in size), underwent 4D US-guide aspiration and there was no evidence of malignancy. One patient suffered from a small subdeltoid ganglion (0.5 cm). After aspiration of about 0.5 cc of jelly-like material, the patient's symptoms were dramatically relieved. There is no recurrence at six months follow up.
Four patients received 4D US-guided injection of a therapeutic drug. All the procedures were performed successfully. One patient with metastatic lymphadenopathy due to a transitional cell carcinoma of the urinary bladder received a direct injection of a chemotherapeutic drug into the diseased lymph node (node size: 2 cm). Another patient received a local injection of corticosteroid into a popliteal cyst (Baker's cyst). The remaining two patients also received corticosteroid injections: one was done in the plantar fascia to treat plantar fasciitis and the other one was done in the subdeltoid bursa to treat subdeltoid bursitis.
Two patients with calcified tendinosis of the supraspinatus tendon of the right shoulder underwent repeat 4D US-guided needle punctures of the calcified plaque. The calcified deposits were resorbed, as was observed on the follow-up US performed two weeks after the procedure.
On the postprocedure follow-up, no major complications or significant minor complications that needed further surgical or invasive procedure management were noted in this study.
Two-dimensional US is commonly used for imaging guidance in interventional radiology because the modern 2D US provides images of adequate quality for most clinical procedures. This modality offers the advantages of real-time capability and wide availability in clinical practice. An experienced radiologist or physician can usually apply this imaging tool without great difficulty. However, real-time 2D US provides only 2D images during image-guided procedures, and it does not offer 3D information about the lesion or the region of interest.
During a 2D US-guided procedure, the operator must sweep the transducer to delineate the spatial relationship between the needle and the target lesion, as is estimated on the serial 2D images. While the needed is being advanced toward or within the lesion, the operator should keep the US transducer steady to enable visualization of the moving needle. Any deviation of the needle out of the plane of the ultrasound beam or any slight movement of the transducer results in the loss of needle visualization. Hence, during the procedure, the operator always advances the needle step by step. 4D US enables real-time, simultaneous monitoring of the advancing needle and of the spatial relationship between the needle and the targeted lesion.
Static 3D US-guided interventional procedures have been applied in several studies and beneficial results were obtained 5-11 for any of the investigations that involved using static 3D US for interventional procedures in deep lesions, such as the ablation of hepatic tumors (8), transjugular intrahepatic portosystemic shunt procedures (5), drainage of intra-abdominal fluid6, and treatment of prostate tumors (9, 11). A few articles refer to the application of 4D US-guided procedure (12). Real-time coronal reformatted images (described as 4D coronal reformatted images) provide an additional image plane to demonstrate lesions, which is not possible with using the 2D US system. With visualization from both the 2D axial images and the 4D coronal reformatted images in the dual-screen format, we are able to obtain information on the needle position in both the axial and coronal planes, and so we were able to direct the needle more precisely during the procedures.
Image-guided interventional procedures in superficial lesions (e.g., those in the breast, the musculoskeletal system and in small areas) rely heavily on US. With the advent of high-resolution US, US-guided procedures for peripheral lesions have become more commonly applied in clinical practice, though a few reports refer to the application of static 3D US in breast biopsy 7, 10. One may propose that a superficial lesion is easier to approach than a deep lesion; hence, the application of 3D US in superficial lesions may not be necessary (12). However, as in our cases, some superficial lesions are small for approaching with a needle, and the target site for the needle in each lesion is even smaller. For instance, for the biopsy of a soft tissue tumor, the optimal site for specimen acquisition is the solid part of the lesion. In the case of lymph node aspiration or biopsy, the optimal site is the cortex of the lymph node. Likewise, with urate deposits in soft tissue, the echogenic crystals may be localized in rather small areas. Therefore, precise needle positioning during aspiration may decrease the false-negative rate for identifying urate crystals. Similarly, during therapeutic drug injection, precise needle positioning for drug injection yields favorable therapeutic effects. In our experience, 4D US aids the physician in directing the biopsy needle as it is advanced toward the desired portion of the target lesions, and this increases the operator's confidence of the needle position during the procedure.
At the initial stage of the procedures, we selected the 2D mode to guide the needles from the skin penetration until the needle tips approached the margin of the lesion (the pre-lesional tract). This was due to the similarity of the echogenicity between the needles and the subcutaneous/muscle layers, which would hinder the visualization of the needles during 3D reconstruction. We recommend 4D US guidance for visualizing the pre-lesional tract only if the echogenicity of the surrounding tissue is low enough to differentiate between the echogenic needle and the tissue within the tract.
In this study, 11 patients who received 4D US-guided core biopsy were found to have soft tissue tumors (seven malignant tumors, two recurrent soft tissue sarcomas and two metastatic carcinomas). The pathologists were able to make the diagnoses on the basis of the representative specimens that were obtained from 4D US-guided biopsy. Furthermore, the diagnoses were identical to the final pathological diagnoses in 10 (90.9%) of the patients. The eight patients who received 4D US-guided core biopsy were found to have non-tumoral lesions. Among them, the pathologic results of core biopsy were identical to the final diagnosis in seven patients (87.5%). As compared with our previous experience, the concordance of the pathological diagnoses between the conventional US-guided core biopsy and the open biopsy for the soft tissue tumors was around 90%, whereas identical pathological results between conventional US-guided core biopsy and open biopsy were observed in 55% (13). Both studies were performed by the same radiologist (H-J.C.); the major difference of the interventional method between both studies was only the use of a different US machine in the current study, i.e., 4D with coronal sections. The operation times were similar between both studies, but different needle sizes were used and there was a different number of patients. Therefore, the significance of the discrepancy between both studies needs further study in the future.
In summary, the patients of this study received 4D US-guided core biopsy and the results had a very high concordance with pathological results and they were identical to the final diagnosis of the surgical specimens. Because accurate pathological diagnosis relies on a sufficient representative specimen obtained from core biopsy, we propose that the additional imaging information from the coronal reformatted 4D US aids the physician in selecting the optimal site for needle placement. This information led to the adequate acquisition of representative specimens for the pathologic examination in our cases.
Conventional 2D US guidance does have several advantages. For example, operators are familiar with the traditional 2D US-guided procedures. In addition, the light transducer used in 2D US is easier to handle during the procedures than the other transduces. Last, adjustment of the angle of the transducer is easier because it is less bulky than the 4D US transducer.
Although our study had a limited number of cases and although we did not compare the results of 2D US- and 4D US-guided procedures, our preliminary results showed the potential of performing 4D US guidance in clinical applications that involved superficial lesions. Further randomized studies for comparing 2D- and 4D US-guided procedures are needed to objectively determine the role of 4D US.
Involuntary motions of patient, such as cardiac motion, arterial pulsation or respiratory motion can result in imaging artifacts when performing 4D US of deep lesions. In our cases, this drawback was minimized because the imaging of superficial lesions is less affected by these involuntary motions. Constant transducer vibration during 4D US acquisition is another major source of image artifacts during procedures (4, 12). However, this did not disturb our procedures nor did it affect the manipulation of the needle. Last, the mechanically driven transducer may be problematic if the operating physician is not comfortable with using such a bulky device, and the fluency of the procedure could potentially be affected. However, we have found that this problem was minimized once the operator adapts to the device.
In conclusion, recent advances in US technique have improved 4D US imaging for its use in clinical practice. Combining the 4D US and 2D US-guided techniques is feasible for performing interventional procedures for superficial lesions.
Figures and Tables
Fig. 1
A 78-year-old woman with a history of surgery for colon cancer developed an enlarged, left inguinal lymph node. During the four-dimensional US-guided biopsy, the needle did not show up on the two-dimensional axial image (left), indicating that it had deviated out of plane. The real-time volume-rendered image in the coronal reformatted plane (right) clearly showed that the needle (arrowheads) was basically in the center of the lesion.
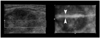
Fig. 2
A 21-year-old man suffering with osteogenic sarcoma in the left distal femur received neoadjuvant chemotherapy and surgical resection of the tumor. An enlarged, left inguinal lymph node was palpated.
A. Color Doppler US showed the enlarged node with a prominent cortex of increased vascularity. The proposed optimal site for the needle aspiration was in the cortical portion of the node (arrowheads).
B. Four-dimensional US-guided fine-needle aspiration was performed. Axial 2D image (left) showed that the needle (arrowheads) had penetrated into the cortex of the node. Real-time volume-rendered imaging in the coronal reformatted plane (right) showed the needle tip (arrow) in the central cortical portion of the node. By combining information from both images, we confirmed that the needle tip was in the central cortical portion of the node in all three orthogonal planes.

Fig. 3
A 41-year-old woman presented with a nodule on the right side of her neck.
A. The image showed the nodule with central cystic change in the right lobe of the thyroid. The solid parts were mainly at the periphery (arrowheads).
B. Four-dimensional US-guided fine-needle aspiration of the nodule was performed. The axial two-dimensional image (left) did not show the needle because it had deviated out of the central plane. The real-time volume-rendered image in the coronal reformatted plane (right) showed the needle tip (arrows) in the periphery of the nodule, which was the desired location for tissue sampling.

References
1. Cho N, Moon WK, Cha JH. Sonographically guided core biopsy of the breast: comparison of 14-gauge automated gun and 11-gauge directional vacuum-assisted biopsy methods. Korean J Radiol. 2005. 6:102–109.
2. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.
3. Lees W. Ultrasound imaging in three and four dimensions. Semin Ultrasound CT MR. 2001. 22:85–105.
4. Downey DB, Fenster A, Williams JC. Clinical utility of three-dimensional US. RadioGraphics. 2000. 20:559–571.
5. Rose SC, Pretorius DH, Nelson TR, Kinney TB, Huynh TV, Roberts AC, et al. Adjunctive 3D US for achieving portal vein access during transjugular intrahepatic portosystemic shunt procedures. J Vasc Interv Radiol. 2000. 11:611–621.
6. Rose SC, Roberts AC, Kinney TB, Pretorius DH, Nelson TR. Three-dimensional ultrasonography for planning percutaneous drainage of complex abdominal fluid collections. J Vasc Interv Radiol. 2003. 14:451–459.
7. Smith WL, Surry KJ, Mills GR, Downey DB, Fenster A. Three-dimensional ultrasound-guided core needle breast biopsy. Ultrasound Med Biol. 2001. 27:1025–1034.
8. Rose SC, Hassanein TI, Easter DW, Gamagami RA, Bouvet M, Pretorius DH, et al. Value of three-dimensional US for optimizing guidance for ablating focal liver tumors. J Vasc Interv Radiol. 2001. 12:507–515.
9. Strasser H, Janetschek G, Horninger W, Bartsch G. Three-dimensional sonographic guidance for interstitial laser therapy in benign prostatic hyperplasia. J Endourol. 1995. 9:497–501.
10. Weismann CF, Forstner R, Prokop E, Rettenbacher T. Three-dimensional targeting: a new three-dimensional ultrasound technique to evaluate needle position during breast biopsy. Ultrasound Obstet Gynecol. 2000. 16:359–364.
11. Chin JL, Downey DB, Mulligan M, Fenster A. Three-dimensional transrectal ultrasound guided cryoablation for localized prostate cancer in nonsurgical candidates: a feasibility study and report of early results. J Urol. 1998. 159:910–914.
12. Won HJ, Han JK, Do KH, Lee KH, Kim KW, Kim SH, et al. Value of four-dimensional ultrasonography in ultrasonographically guided biopsy of hepatic masses. J Ultrasound Med. 2003. 22:215–220.
13. Liu JC, Chiou HJ, Chen WM, Chou YH, Chen TH, Chen W, et al. Sonographically guided core needle biopsy of soft tissue neoplasms. J Clin Ultrasound. 2004. 32:294–298.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download