Abstract
Objective
To evaluate the effect of thin overlapping reconstruction on the attenuation of small (≤ 3 cm) renal cysts in the nephrographic phase of multidetector CT (MDCT).
Materials and Methods
We scanned a phantom kidney containing spheres of various sizes (10, 20, and 30 mm) using both 4- and 16-channel MDCT scanners, and reconstructed images with various slice thickness (T, mm) and intervals (I, mm). The attenuation increase (AI) was measured for each sphere in 240-HU diluted solution of contrast material and compared with the attenuation in 35-HU solution.
Results
On the 4-channel MDCT, thin overlapping reconstruction (T/I = 3/1, compared with 5/5) lowered the AI as much as 17 HU in the 10 mm-sphere and 6 HU in the 20 mm-sphere (p < 0.05). Thin slicing alone was also effective; however overlapping alone was not. On the 16-channel MDCT, AI in the 10 mm-sphere was significantly lower than on the 4-channel MDCT with T/I = 5/5 (p < 0.05), however thinner slicing or overlapping did not affect the attenuation significantly in all of the spheres.
The diagnosis of renal cysts is usually made by sonography. However, some cysts show atypical sonographic features and CT is required for further evaluation to rule out tumors. Many renal cysts are also incidentally found on CT. In order to differentiate renal cysts from tumors on CT, the most important factor is the absence of contrast enhancement (1-3).
However, many factors affect CT attenuation and result in falsely elevated attenuation of a cyst in a postcontrast scan; this makes the differentiation of a cyst from a poorly enhancing tumor difficult (4, 5) (Fig. 1). Partial volume averaging is a well known factor that can contribute to the difficulty of differentiation as well. In addition, beam hardening can also alter attenuation values, resulting in a 'pseudoenhancement' of renal cysts. Pseudoenhancement is thought to be resulted from inadequate correction of beam hardening due to strongly enhanced renal parenchyma by CT imaging reconstruction algorithm (6).
Either by partial volume averaging or pseudoenhancement, the degree of falsely increased attenuation is higher in smaller cysts and when the renal parenchyma is more strongly enhanced (7). Renal parenchyma shows maximum enhancement in the nephrographic phase of helical CT scanning, where renal parenchymal lesions can best be detected (8). The upper limit of pseudoenhancement in this phase has been accepted as 10 HU on a single detector CT (SDCT); this means true enhancement should be considered if the attenuation increases above this limit (6, 7, 9-11). SDCT is now being replaced by the multidetector CT (MDCT). Recent studies have reported a higher degree of pseudoenhancement with the MDCT than on the SDCT. Therefore, this issue becomes more important in the MDCT era (12, 13).
One of the major advantages of MDCT is that retrospective reconstruction of images, with thinner slicing or overlapping, is possible if the raw image data is available. A recent clinical study reported that this retrospective thin overlapping reconstruction could help characterize small (≤ 3 cm) renal masses by decreasing attenuation in cysts (14). However, they evaluated only the combined effect of thin slicing and overlapping on a 4-channel MDCT scanner.
The purpose of this study was to evaluate the effect of thin overlapping reconstruction on the attenuation of small (≤ 3 cm) renal cysts in the nephrographic phase of the MDCT. We used both the 4- and 16-channel MDCT scanners, and analyzed both the combined and separate effects of thin slicing and overlapping. We performed a phantom study because a patient cannot be scanned with two different scanners at the same time.
Three spheres of different sizes (10, 20, and 30 mm in diameter) were made with a latex balloon filled with distilled water. A 9×15 cm plastic cylinder was used as a kidney phantom (Fig. 2). The spheres were suspended in the center of the cylinder along the longitudinal axis at regular intervals by hanging them on the inner wall with threads. The cylinder was filled with 370 mgI/ml of iodinated contrast material (Ultravist [iopromide]; Berlex Laboratory, Wayne, NJ) diluted in water and air bubbles were removed as completely as possible through a small hole on the top of the cylinder.
The concentration of the solution in the cylinder was adjusted to 240 Hounsfield units (HU), which is similar to the attenuation of enhanced renal parenchyma in the nephrographic phase. Another solution was prepared with a concentration corresponding to 35 HU, which is similar to the attenuation of nonenhanced renal parenchyma. The phantom kidney was placed in the center of a rectangular water bath (25×30×35 cm), which served as a phantom abdomen.
The phantom was scanned using a 35-HU solution followed by a 240-HU solution during the same CT scanning session. Attenuation increase (AI) was measured in each sphere in the 240-HU solution and compared with the attenuation in the 35-HU solution. To avoid a bias due to the temporal variations of the CT scanners, we performed these scans twice with a one-month interval.
A 4-channel MDCT scanner (Picker MX 8000; Marconi Medical Systems, Cleveland, OH) and a 16-channel MDCT scanner (Sensation 16; Siemens, Inc., Forchheim, Germany) were used. The detector configuration was 2.5 mm×4 for the 4-channel MDCT and 0.75 mm×16 for the 16-channel MDCT; these are the usual settings for the kidney CT protocol at our hospital. The pitch was 1.25, field of view (FOV) was 123 mm, and other parameters were 200 mA and 120 KVp. These settings remained constant throughout the study.
Axial images were reconstructed with various combinations of slice thickness (T, mm) and intervals (I, mm). The T/I combinations used were 5/5, 5/3, 5/1, 3/3, and 3/1. The results were analyzed according to sphere size and type of MDCT scanner.
At the workstation, we placed a region of interest (ROI) in the center of each sphere and tried to maintain the size of the ROI for the same-sized spheres. The approximate areas of the circles were 30, 140, and 350 mm2 for 10, 20, and 30 mm cysts, respectively. The HU of each sphere was measured five times and the averages and standard deviations (SD) were calculated. Statistical analysis was performed using the Kruskal-Wallis test and the Wilcoxon rank sum test.
The resulting detailed values are listed in Table 1. Both on the 4- and 16-channel MDCT, the AI was highest in the 10 mm-sphere and decreased as the size of the sphere increased. With T/I = 5/5, the AI in the 10 mm-sphere was significantly lower on the 16-channel MDCT than on the 4-channel MDCT (p < 0.05). However, there was no significant difference observed in the larger spheres or with other T/Is.
On the 4-channel MDCT, thinner slicing alone lowered AI of the 10 mm-sphere significantly (T/I = 5/5 vs. 3/3, p < 0.05); however, overlapping alone did not (T = 5/5 vs. 5/3 and 5/1, p > 0.05). Overlapping was effective only with thinner slicing (T = 3/3 vs. 3/1, p < 0.05). The combined effect of thin slicing and overlapping, on lowering AI in this sphere, was as much as 17 HU. This effect of thin overlapping reconstruction was also significant in the 20 mm-sphere (as much as 5 HU, p < 0.05) but insignificant in the 30 mm-sphere. For the 16-channel MDCT, neither thinner-slicing nor overlapping reconstruction lowered AI significantly regardless of sphere size or T/Is.
Increased attenuation in simple renal cysts on postcontrast CT scanning is an important problem for accurate characterization of small renal masses (Fig. 1). 'Pseudoenhancement' is now generally accepted as a term that describes this phenomenon. Many studies have worked on this issue to improve our understanding of this problem; the current explanation is an inadequate correction of beam hardening by the reconstruction algorithm as the cause for pseudoenhancement. However, in small cysts, partial volume averaging is also partly responsible (6).
The upper limit of increased attenuation in a renal cyst on postcontrast helical CT scan (SDCT) is now generally accepted as 10 HU based on the results of many phantom and clinical studies (6, 7, 9-11) One phantom study suggested that this limit should be raised to 20 HU on MDCT due to the different reconstruction algorithm used (12). However, a recent clinical study with MDCT showed that changes in attenuation of pathologically-proven renal cysts, at different scanning phases of triphasic MDCT, did not exceed 10 HU (15).
These previous studies focused on determining the upper limit of pseudoenhancement. However, it may be more important, in the clinical setting, to determine the appropriate method for acquiring images that minimizes falsely elevated attenuation, in indeterminate lesions, while limiting exposure to radiation. In previous phantom studies on MDCT, many CT parameters were verified for their effect on pseudoenhancement including: slice thickness, pitch and detector configuration. However, only slice thickness in small lesions (less than 10 mm) showed a considerable effect on attenuation (12, 13). Prior to our current study, we confirmed that changes in pitch or detector configuration did not cause significant changes in attenuation of spheres with MDCT scanners.
One of the major advantages of MDCT is that retrospective reconstruction of thin overlapping images is possible when data sets from a scan with thin collimation are available; using this approach a recent clinical study improved the characterization of small (≤ 30 mm) renal masses by MDCT (14). They reconstructed images with 3-mm thickness and 50% overlap and compared them with images 5-mm thickness and no overlap on a 4-channel MDCT scanner (detector collimation = 2.5 mm). Their findings were based on results from decreased attenuation in small cysts by thin overlapping reconstruction. They used only a 4-channel MDCT scanner, but the result may be different on a 16-channel MDCT because of the different reconstruction algorithm and a much narrower detector-collimation (16). Furthermore, the investigators only examined the combined effect of thin slicing and overlapping, so the effect of thin slicing or overlapping alone was not evaluated. Therefore, we pursued study of thin slicing and overlapping as independent factors in a phantom study.
Our results showed that thin overlapping reconstruction was effective in decreasing the degree of AI only in spheres ≤ 20 mm on a 4-channel MDCT. Thin slicing alone was effective but overlapping alone was not. On the 16-channel MDCT, AI in the 10-mm sphere was significantly lower than on the 4-channel MDCT; thin overlapping reconstruction did not affect AI significantly in any of the spheres studied. As previously mentioned, partial volume averaging and pseudoenhancement due to beam hardening are the two most important causes of AI in small renal cysts on postcontrast CT scanning. Therefore, we tried to explain our results in regard to these two causes of AI.
Regarding the effect of partial volume averaging, cylinders are ideal vessels for phantom cysts to eliminate this effect (6, 7). We used spheres because our goal was not only to analyze the pseudoenhancement but to simulate the real renal cyst to determine the actual degree of AI; for potential practical use in the clinical setting. For the spheres, a partial volume effect can be considered minimal if the slice thickness is smaller than half of the diameter of the sphere (10). The slice thickness we applied (5 and 3 mm) was the same or smaller than the diameter of the smallest sphere (10 mm). The effect of the thin overlapping reconstruction was also significant in the larger (20 mm) sphere on the 4-channel MDCT. Therefore, our result cannot be explained by partial volume averaging alone. The lower AI in the 10 mm-sphere on the 16-channel MDCT compared to the 4-channel MDCT with the same slice thickness (5 mm) also cannot be explained in this way.
In regard to pseudoenhancement, it is possible that the degree of pseudoenhancement might be different in comparison of the 4- and 16-channel MDCT because the reconstruction algorithm is different for each scanner (16). The reconstruction algorithm has been considered as the most important factor for the differences in pseudoenhancement noted in prior studies, and is likely responsible, in part, for the results observed. The detector collimation is another factor to consider for interpretation of our results. Abdulla et al. reported that differences of detector collimation on the 4-channel MDCT did not affect the attenuation of cysts (13). However, they compared detector collimations of 1.25 and 2.5 mm, a difference smaller than what we used (0.75 vs. 2.5 mm). Therefore, this difference may have affected the result.
Our phantom was a simple model and has limitations in reflecting the in vivo state of a patient. However, many previous studies using phantoms similar to ours also have the same limitation; although there is one study that has performed a phantom study using an anthropomorphic body CT that better simulated the human abdomen (10). We plan future studies with a phantom reflecting the in vivo human state more closely.
We used CT scanners manufactured by different vendors, which might also affect the results. . Different degrees of pseudoenhancement have been reported from helical CT scanners from different vendors; this may be explained by the different imaging algorithms used (9, 10). Methods used for the detector array in MDCT scanners may also be different from different vendors; different degrees of pseudoenhancement have been reported in two 4-channel MDCT scanners that use different detector-array methods (13). However, the difference in the 10-mm sphere with T/I = 5/5 was only statistically significant in comparisons between the 4- and 16-channel MDCT in our study. If the effect was based on different vendors, there should have also been significant differences in other spheres or with other T/Is. Therefore, this difference was unlikely to have affected our findings. We also plan future studies on this topic using MDCT scanners from the same vender.
In conclusion, thin overlapping reconstruction can be an effective method used for minimizing falsely elevated attenuation of renal cysts ≤ 20 mm in the nephrographic phase of 4-channel MDCT. Overlapping alone is not effective. When the standard kidney CT protocol setting (T/I = 5/5) is applied, using a 16-channel MDCT can lower the attenuation in 10 mm-sized cysts.
Figures and Tables
Fig. 1
Representative cases showing the importance of falsely increased attenuation in small renal cysts in the nephrographic phase of MDCT. All scans were performed with the 16-channel MDCT scanner used in the phantom study. Scans in the nephrographic phase were obtained 150 seconds after intravenous injection of contrast material (same material used in the phantom study). Slice thickness / interval was 5 mm/5 mm.
A-C. An 1-cm-sized lesion (arrows) in the kidney shows 19 HU on the precontrast scan (A) and 37 HU in the nephrographic phase (B). On ultrasonography (C), this lesion was identified as a cyst.
D-F. A less than 1-cm-sized lesion (arrows) in the kidney of another patient is 15 HU on the precontrast scan and 44 HU in the nephrographic phase. On ultrasonography, this lesion appears as an echogenic solid mass. A collecting duct carcinoma was confirmed after nephrectomy.
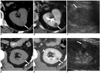
Fig. 2
CT images of the phantom.
A. Scannogram of the kidney phantom shows spheres of various sizes (10, 20, and 30 mm) suspended in the center of the phantom along the longitudinal axis. The kidney phantom was filled with contrast material of 240 HU to simulate enhanced renal parenchyma in the nephrographic phase.
B. Axial CT scan at the mid level of the phantom shows the 20 mm-sized sphere surrounded by contrast material (T/I = 3/3).

References
1. Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986. 158:1–10.
2. McClennan BL, Stanley RJ, Melson GL, Levitt RG, Sagel SS. CT of the renal cyst: is cyst aspiration necessary? AJR Am J Roentgenol. 1979. 133:671–675.
3. Sagel SS, Stanley RJ, Levitt RG, Geisse G. Computed tomography of the kidney. Radiology. 1977. 124:359–370.
4. Bosniak MA, Rofsky NM. Problems in the detection and characterization of small renal masses. Radiology. 1996. 198:638–641.
5. Siegel CL, Fisher AJ, Bennett HF. Interobserver variability in determining enhancement of renal masses on helical CT. AJR Am J Roentgenol. 1999. 172:1207–1212.
6. Maki DD, Birnbaum BA, Chakraborty DP, Jacobs JE, Carvalho BM, Herman GT. Renal cyst pseudoenhancement: beam-hardening effects on CT numbers. Radiology. 1999. 213:468–472.
7. Coulam CH, Sheafor DH, Leder RA, Paulson EK, DeLong DM, Nelson RC. Evaluation of pseudoenhancement of renal cysts during contrast-enhanced CT. AJR Am J Roentgenol. 2000. 174:493–498.
8. Birnbaum BA, Jacobs JE, Langlotz CP, Ramchandani P. Assessment of a bolus tracking technique in helical renal CT to optimize nephrographic phase imaging. Radiology. 1999. 211:87–94.
9. Gokan T, Ohgiya Y, Munechika H, Nobusawa H, Hirose M. Renal cyst pseudoenhancement with beam hardening effect on CT attenuation. Radiat Med. 2002. 20:187–190.
10. Birnbaum BA, Maki DD, Chakraborty DP, Jacobs JE, Babb JS. Renal cyst pseudoenhancement: evaluation with an anthropomorphic body CT phantom. Radiology. 2002. 225:83–90.
11. Bae KT, Heiken JP, Siegel CL, Bennett HF. Renal cysts: is attenuation artifactually increased on contrast-enhanced CT images? Radiology. 2000. 216:792–796.
12. Heneghan JP, Spielmann AL, Sheafor DH, Kliewer MA, DeLong DM, Nelson RC. Pseudoenhancement of simple renal cysts: a comparison of single and multidetector helical CT. J Comput Assist Tomogr. 2002. 26:90–94.
13. Abdulla C, Kalra MK, Saini S, Maher MM, Ahmad A, Halpern E, et al. Pseudoenhancement of simulated renal cysts in a phantom using different multidetector CT scanners. AJR Am J Roentgenol. 2002. 179:1473–1476.
14. Jinzaki M, McTavish JD, Zou KH, Judy PF, Silverman SG. Evaluation of small (3 cm) renal masses with MDCT: benefits of thin overlapping reconstructions. AJR Am J Roentgenol. 2004. 183:223–228.
15. Chung EP, Herts BR, Linnell G, Novick AC, Obuchowski N, Coll DM, et al. Analysis of changes in attenuation of proven renal cysts on different scanning phases of triphasic MDCT. AJR Am J Roentgenol. 2004. 182:405–410.
16. Kalra MK, Maher MM, D'Souza R, Saini S. Multidetector computed tomography technology: current status and emerging developments. J Comput Assist Tomogr. 2004. 28:S2–S6.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download