Abstract
Objective
We wanted to evaluate whether tumors located in a segmental border zone are predisposed to local recurrence after performing segmental transarterial chemoembolization for hepatocellular carcinoma.
Materials and Methods
Seventy-three hepatocellular carcinoma nodules were retrospectively analyzed for local tumor recurrence after performing segmental transarterial chemoembolization by using follow-up CT studies (median follow-up period: 20 months, range: 4-77 months). The tumors were divided into two groups according to whether the lesions were located at the segmental border zone (Group I) or not (Group II). Comparison of the tumor characteristics and chemoembolization methods between the two groups was performed using the chi-square test. The local recurrence rates were compared by Kaplan-Meyer method and analyzed with the log rank test.
Results
Local tumor recurrence occurred for 25 hepatocellular carcinoma nodules (42.9%). The follow-up periods, tumor characteristics and chemoembolization methods between Groups l and ll were comparable. The local recurrence rate was 64.0% (16/25) in Group I and 18.8% (9/48) in Group II. The difference was statistically significant on the univariate and multivariate analyses (p = 0.000 for both).
Surgical resection or transplantation has been considered the treatment of choice for hepatocellular carcinoma (HCC) in the patients who are candidates for surgery (1, 2). However, transcatheter arterial chemoembolization (TACE) is now widely used in the cases of surgically unresectable HCC (3-8). The prognostic factors of local HCC tumor recurrence after TACE are well known, such as the tumor size or the homogeneity of iodized oil uptake (9, 10). However, at our medical center, we found that tumors located in the segmental border zone had a tendency to show more frequent local tumor recurrence after TACE. To the best of our knowledge, location of tumor in the segmental border zone has not been reported as a significant prognostic factor of local tumor recurrence after segmental TACE in any of the English medical literature. In this study, we tried to analyze whether tumor located in the segmental border zone was significantly associated with local HCC tumor recurrence after performing segmental TACE.
From July 1998 to August 2004, 272 patients with HCC were referred to our center for TACE. Among them, 107 patients who had hypervascular tumors observed on their hepatic angiogram and less than four nodules were treated with segmental TACE. Among these 107 patients, four patients who had extrahepatic tumor invasion and three patients who had evidence of hepatic or portal vein tumor thrombosis were all excluded from the analysis. Another 15 patients were eventually lost during follow-up. Among the remaining patients, 27 patients displayed residual enhanced areas within or around the tumors on the one month follow-up CT imaging, and they subsequently received other treatments for their liver masses such as repeated TACE or radiofrequency tissue ablation therapy (RFAT); these 27 patients were also excluded from the analysis. So finally, 58 patients with 73 HCC nodules were included in our study. The median follow-up period was 20 months (range: 4-77 months).
All the patients except one were males. Their ages ranged from 44 to 77 years (mean±SD: 62.2±9.3 years). The tumor size ranged from 1.0 cm to 7.3 cm (mean±SD: 3.0±1.4 cm). The other characteristics are shown in Table 1.
The diagnosis of HCC was proved histopathologically for seven patients. For the other patients, the diagnosis was established on the basis of the characteristic imaging findings on the three-phase helical CT and conventional angiography and/or by the presence of elevated levels of tumor markers in the blood serum (alpha-fetoprotein level > 200 ng/mL) (11). In the vast majority of patients, the etiology of cirrhosis was chronic viral hepatitis B or hepatitis C (Table 1).
All patients had enhanced dynamic CT images taken within four weeks prior to TACE. All the TACE procedures were performed by an interventional radiologist with over five years experience (Y.K.C). Hepatic angiography was performed using 5 Fr angiographic catheters; this was followed by superselection of the segmental arterial feeders using a microcatheter (Microferret®; Cook, Bloomington, IN). We then administered iodized oil-doxorubicin hydrochloride (Adriamycin; Kyowa Hakko Kogyo, Tokyo, Japan) emulsion into the feeders. The volume of iodized oil ranged from 3 to 10 ml. Once the flow became sluggish, gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, MI) that were mixed with contrast material (Iopromide; Schering, Germany) were administered into the feeders until the blood flow stopped completely. While performing segmental TACE, attempts were made to completely occlude the arterial feeders. A small amount of saline solution was then slowly injected to confirm the complete occlusion of the segmental arterial feeder. If the retained contrast media was partially washed out after the saline injection, additional gelatin sponge particles were infused until complete stasis of flow was obtained.
Among the 73 nodules, four nodules were supplied by two segmental arteries, as was determined on selective angiography with using a microcatheter. In these circumstances, both segmental feeding arteries were embolized.
The local recurrence rate was analyzed for nine possible prognostic factors: patient age, hepatitis C infection, the modified Child-Pugh classification, the number of tumors, size of the tumor nodule, the serum alpha-fetoprotein level, homogeneity of the iodized oil accumulation within the nodule, tumor location in a segmental border zone and the tumor growth pattern.
The number of tumors was determined on the preembolization CT. Tumor size was determined as the maximal diameter of the nodule that was measured on the preembolization CT.
The CT examinations were performed with an 8-slice multidetector CT scanner (Lightspeed; GE Medical Systems, Milwaukee, WI) with 5-mm collimation and 17.5-mm/sec table speed, or with a single-detector helical scanner (Prospeed Advantage; GE Medical Systems, Milwaukee, WI) with 10-mm collimation and a 10-mm/sec table speed.
The border zone between the hepatic segments was determined by tracing the portal venous tree on the portal phase scan of the spiral CT (12, 13). The segmental border zone was defined as an area without traceable portal veins between the hepatic segments on the CT scan. Segmental border zone lesions were defined as lesions crossing the imaginary border between the hepatic segments.
All the patients underwent both non-enhanced and contrast-enhanced three-phase helical CT four weeks after TACE. The pattern of iodized oil accumulation in the masses was evaluated with using the four-week follow-up CT scan. When the tumor nodules showed compact iodized-oil accumulation without any defect, they were classified as a "homogeneous" pattern. Otherwise, they were classified as an "inhomogenous" pattern.
When the tumor nodules showed sharp definition from the surrounding liver parenchyma without any evidence of adjacent satellite nodules, they were classified as "nodular" tumors. Otherwise, they were classified as "nonnodular" tumors.
Residual viable tumor was judged to be present when an enhanced portion was seen within or around of the original mass on the one-month follow-up CT scan. If no definite evidence of residual tumor was noted on this one-month follow-up CT, then 3-phase contrast-enhanced CT was performed at 3- or 4-month intervals thereafter. Local tumor recurrence was judged to be present when we observed disappearance of iodized oil from the lesion, or when an enhanced portion was seen within or at the margin of the original mass on the next follow-up CT scan that was done after the first one-month follow-up CT scan. For the recurred tumors, additional therapies such as TACE or RFAT were performed.
Two abdominal radiologists with five and four years of experience, respectively, (Y.O.P., Y.S.A.) interpreted the CT images; they independently determined the segmental zonal anatomy, and they were kept unaware of whether the tumor showed local tumor recurrence on the follow-up CT images. Final decisions were reached by consensus.
Univariate and multivariate analysis was performed using Kaplan-Meyer estimation and Cox proportional hazard model for the nine possible prognostic factors of local tumor recurrence. The parameters that proved to be significant on the univariate analysis were then subsequently tested with the multivariate Cox proportional hazard model.
Specifically, the tumors were divided into two groups according to whether the lesions were located at the segmental border zone (Group I) or not (Group II). Tumor characteristics such as tumor size, the tumor growth pattern, the iodized oil uptake pattern on the one-month follow-up CT imaging and the follow-up periods were compared between the two groups. The local tumor recurrence rates were also compared between the two groups.
Comparison of the tumor characteristics and TACE methods between the two groups were performed using the Chi-square test. The local recurrence rate was compared between the two groups by the Kaplan-Meyer method and it was analyzed with the log rank test.
P-values less than 0.05 were considered statistically significant. The SPSS software package (Version 10.0; SPSS Inc., Chicago, IL) was used for the statistical analysis.
The median CT follow-up period was 20 months (range: 4-77 months). Local tumor recurrence occurred for 25 HCC nodules among the 73 nodules (34.2%). Kaplan-Meyer estimation and log-rank testing revealed that among the nine possible prognostic factors, tumor location in a segmental border zone and the iodized oil uptake pattern within the nodule were significantly associated with local recurrence (p = 0.000 for both). However, other factors such as tumor size, the tumor growth pattern, patient age, the modified Child-Pugh classification, hepatitis C or serum alpha-fetoprotein level were not significantly associated with local tumor recurrence (Table 2). Multivariate analysis also revealed that the iodized oil uptake pattern and a tumor location in a segmental border zone had a statistically significant adverse effect on local tumor recurrence (p = 0.000 and 0.001, respectively).
There were 25 lesions in a segmental border zone (Group I) and 48 lesions that were not in a segmental border zone (Group II) (Figs. 1, 2). The comparison of tumor characteristics and follow-up periods between the two groups is presented in Table 3. Although the average tumor size in Group I tended to be larger than that of Group II (p = 0.122), there was no statistically significant difference in the iodized-oil accumulation pattern, the tumor growth pattern and the median follow-up period between the two groups (Table 3).
Among the 12 small tumors ≤ 3 cm in size in Group I, five showed homogeneous iodized oil accumulation and the other seven showed inhomogeneous iodized oil accumulation. Among the 13 larger tumors > 3 cm in size in Group I, eight showed homogeneous iodized oil accumulation and the other five showed inhomogeneous iodized oil accumulation. Among the 32 small tumors ≤ 3 cm in size in Group II, 20 tumors showed homogeneous iodized oil accumulation and the other 12 tumors showed inhomogeneous iodized oil accumulation. Among the 16 larger tumors > 3 cm in size in Group II, 11 tumors showed homogeneous iodized oil accumulation and the other five showed inhomogeneous iodized oil accumulation. The Chi-square test revealed no statistically significant correlation between the tumor size and the iodized oil accumulation patterns in each group.
The period of local tumor recurrence after TACE ranged from three months to 32 months (mean±SD: 9±7 months). The median period of local recurrence was seven months. In Group I, the median period of local recurrence was seven months (range: 3-20 months). In Group II, the median period of local recurrence was eight months (range: 4-32 months). In Group I, the 1-year and 3-year estimated local tumor recurrence rates were approximately 51.5% and 79.2%, respectively. In Group II, the 1-year and 3-year estimated local tumor recurrence rates were 18.6% and 34.9%, respectively. The difference in the local tumor recurrence rates between the two groups was statistically significant (p = 0.000) (Fig. 3), which means Group I showed a higher local tumor recurrence rate compared to Group II. For the Group II lesions with homogeneous iodized oil accumulation, the estimated 1-year and 3-year local tumor recurrence rates were 6.5% and 22.0%, respectively.
Among the 25 patients with local tumor recurrence after segmental TACE, 14 patients underwent follow-up hepatic angiography. Among the 14 patients, only three patients showed reopening of the originally occluded segmental arterial feeders, and all the other patients showed no evidence of reopening of the originally occluded segmental arteries (Fig. 2).
To treat the local recurred tumors, we performed radiofrequency tissue ablation therapy for six nodules, TACE for 15 nodules and surgical resection for one nodule. The other three nodules were conservatively treated because of poor liver function or the patients refused further treatment. Fifteen of the 22 recurred tumors that were treated showed no evidence of residual viable tumor on the follow-up CT scan. The estimated 1-year and 3-year overall survival rates were 81.6% and 68.0%, respectively. At the end of the study, 25 patients had expired. And among them, eight patients died of recurrent HCC.
The risk factors of local HCC tumor recurrence after segmental TACE are well known (9, 10). The same as for other studies, the present study also showed that the local tumor recurrence rate was higher for the tumors showing inhomogeneous iodized oil uptake. However, it has not been clearly reported until now whether or not tumor location in a segmental border zone is associated with local tumor recurrence after performing segmental TACE.
In this study, tumor location in a segmental border zone was proved to be another risk factor for local tumor recurrence after segmental TACE for HCC by both the univariate and multivariate analyses. Most of the recurrent tumors were supplied by feeders from the adjacent segmental arteries, and most of the originally occluded segmental arteries had not reopened, as was seen on the follow-up hepatic angiograms.
One of the reasons why Group I showed a higher local recurrence rate may be that there were more chances for missing the fine arterial feeders from the adjacent segmental arteries. The other possible reason may that the recurred tumors might have survived via their portal vein supply after performing segmental TACE (14). The collateral vessels would probably develop from the adjacent segmental arteries for these tumors, but the shortest distance between Group I and adjacent segmental arteries was shorter than that between Group II and the adjacent segmental arteries. This would have caused earlier tumor recurrence for Group I.
In this study, tumor size was not associated with local tumor recurrence. This finding stands in contrast to other previous studies (9, 10), and it may have been caused by the fact that only the tumors treated with segmental TACE were included in our study, and relatively strict measures of segmental tumor feeder occlusion were applied in most of the cases. In this study, the tumor growth pattern was not also significantly associated with local tumor recurrence. This finding may have been caused by the fact that the segmental arteries were completely occluded in most of the cases.
The limitations of this study were as follows. First, the segmental anatomy was not determined by CT arterioportography. However, the decision on the number of segmental lesions was made by consensus of two experienced abdominal radiologists with tracing the portal venous tree on the portal phase scan of the spiral CT. Second, the adjacent segmental or subsegmental arteries of the tumors were not embolized if definite hypervascular tumor staining was not noted on the hepatic angiogram. Employing a unified helical CT/angiography system may be helpful for this modified embolization procedure (10). However, more radical treatment might further deteriorate the hepatic function, and the clinical benefit of this technique needs to be studied by conducting future controlled trials. Also, the other ablative therapies such as RFAT may have been preferable for treating the lesions in Group I. Third, any enhanced area on the follow-up CT imaging might have been masked by overlying iodized oil accumulation in the tumor.
In conclusion, the local HCC tumor recurrence rate was higher for the nodules located in the segmental border zone after treatment with segmental TACE as compared to the recurrence rate for the nodules located inside hepatic segments.
Figures and Tables
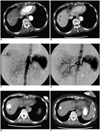 | Fig. 1A 73-year-old woman with hepatocellular carcinoma.
A. Pre-embolization CT imaging on the arterial phase reveals a well-defined enhanced mass measuring 2.9 cm in segment 8 of the liver (arrowhead). The low density lesion in the left lobe dome area is a benign cystic lesion without any interval change over a year (arrow).
B. Pre-embolization CT imaging on the portal venous phase reveals the same mass with heterogeneous contrast enhancement (arrowhead).
C. Pre-embolization hepatic angiogram shows the mass with heterogeneous hypervascularity (arrowhead).
D. Post-embolization hepatic angiogram shows the mass without evidence of hypervascular tumor staining. Note that the segmental arterial feeder was also completely occluded.
E. One-month follow-up CT imaging shows the mass with inhomogeneous iodized oil accumulation (arrowhead).
F. Seven-month follow-up CT imaging reveals the mass with shrinkage (arrowhead). No definite evidence of local tumor recurrence was noted.
|
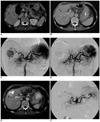 | Fig. 2A 70-year-old man with hepatocellular carcinoma.
A. Pre-embolization CT imaging on the portal phase reveals a large well-defined mass measuring 5.1 cm in the S5 segment (arrow). Portal and delayed phase CT imaging (not shown) revealed delayed marginal rim enhancement, which is a typical finding of hepatocellular carcinoma.
B. Pre-embolization CT imaging on the portal phase at the level of segmental border zone area between segments 5 and 8. Note that the upper portion of the tumor is also seen at this level (arrowheads). Therefore, this tumor was regarded as located in the segmental border zone between S5 and S8.
C. Pre-embolization hepatic angiogram shows the mass with hypervascularity (arrowhead). Note the segmental arterial feeder of the tumor (arrowhead). A minor blood supply to the tumor from the adjacent segmental artery was also confirmed (not shown).
D. Post-embolization hepatic angiogram reveals no evidence of residual hypervascular tumor staining. Note that the right hepatic artery is completely occluded.
E. One-month follow-up CT imaging shows the mass with inhomogeneous iodized oil accumulation at the segmental border zone of S5 and S8 (arrowhead). The high density area within the tumor was also seen on the precontrast CT imaging (not shown here).
F. Seven-month follow-up hepatic angiogram reveals the tumor with local recurrence. Note that the original segmental feeders are completely occluded (arrowheads). Chemoembolization was performed for this mass.
|
 | Fig. 3Comparison of local tumor recurrence rates between tumors located in segmental border zones (n = 25) and those located inside the hepatic segments (n = 48). Tumors located in segmental border zones showed earlier local tumor recurrence compared to those tumors located inside hepatic segments on the univariate and multivariate analyses with using Kaplan-Meyer estimation and the log rank test (p = 0.000 for both). |
References
1. Colella G, Bottelli R, De Carlis L, Sansalone CV, Rondinara GF, Alberti A, et al. Hepatocellular carcinoma: comparison between liver transplantation, resective surgery, ethanol injection, and chemoembolization. Transpl Int. 1998. 11:S193–S196.
2. Segawa T, Izawa K, Tsunoda T, Kanematsu T, Shima M, Matsunaga N, et al. Evaluation of hepatectomy in small hepatocellular carcinoma: comparison with transcatheter arterial embolization therapy. Nippon Geka Gakkai Zasshi. 1992. 93:1095–1099.
3. Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oil chemoembolization of hepatocellular carcinoma. Radiology. 1989. 170:783–786.
4. Bronowicki JP, Vetter D, Dumas F, Boudjema K, Bader R, Weiss AM, et al. Transcatheter oil chemoembolization for hepatocellular carcinoma: A 4-year study of 127 French patients. Cancer. 1994. 74:16–24.
5. Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, et al. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990. 13:140–145.
6. Takayasu K, Suzuki M, Uesaka K, Muramatsu Y, Moriyama N, Yoshida T, et al. Hepatic arterial embolization for inoperable hepatocellular carcinoma: prognosis and risk factors. Cancer Chemother Pharmacol. 1989. 23:S123–S125.
7. Yamada R, Kishi K, Sonomura T, Tsuda M, Nomura S, Satoh M. Transcatheter arterial embolization in unresectable hepatoellular carcinoma. Cardiovasc Intervent Radiol. 1990. 13:135–139.
8. Pelletier G, Rohe A, Ink O, Anciaux ML, Derhy S, Rougier P, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990. 11:181–184.
9. Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, et al. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001. 176:681–688.
10. Maeda S, Fujiyama S, Tanaka M, Ashihara H, Hirata R, Tomita K. Survival and local recurrence rates of hepatocellular carcinoma patients treated by transarterial chemolipiodolization with and without embolization. Hepatol Res. 2002. 23:202–210.
11. Nguyen M, Garcia R, Simpson P, Wright T, Keeffe E. Racial differences in effectiveness of alpha-fetoprotein for the diagnosis of hepatocellular carcinoma in hepatitis C cirrhosis. Hepatology. 2002. 36:410–417.
12. Fischer L, Cardenas C, Thorn M, Benner A, Grenacher L, Lehnert T, et al. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. J Comput Assist Tomogr. 2002. 26:962–967.
13. Choi D, Choo SW, Lim JH, Lee SJ, Do YS, Choo IW. Opacification of the intrahepatic portal veins during CT hepatic arteriography. J Comput Assist Tomogr. 2001. 25:218–224.
14. Choi SH, Chung JW, Lee HS. Hepatocellular carcinoma supplied by portal flow after repeated transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2003. 181:889–890.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download