Abstract
Objective
We wanted to investigate the prevalence and causative factors of extrahepatic arterial blood supply to hepatocellular carcinoma (HCC) at its initial presentation and during chemoembolization.
Materials and Methods
Between February 1998 and April 2000, consecutive 479 patients with newly diagnosed HCC were prospectively enrolled into this study. A total of 1629 sessions of transcatheter arterial chemoembolization (TACE) were performed in these patients (range: 1-15 sessions; mean: 3.4 sessions) until April 2004. For each TACE procedure, we determined the potential extrahepatic collateral arteries (ExCAs) depending on the location of the tumor, and we performed selective angiography of all suspected collaterals that could supply the tumor. The prevalence of ExCAs and the causative factors were analyzed.
Results
At initial presentation, 82 (17%) of these 479 patients showed 108 ExCAs supplying tumors. Univariate analysis showed that tumor size (p < 0.01), patient age (p = 0.02), a surface location (p < 0.01), and a bare area location (p < 0.01) were significantly associated with the presence of ExCAs. Multiple logistic regression analysis showed that only tumor size was predictive of ExCA formation (p < 0.01, odds ratio = 1.737, confidence interval: 1.533 to 1.969). During repeated TACE sessions, 97 additional ExCAs were detected in 70 (14%) patients. The cumulative probability of ExCAs in patients with a large tumor (≥ 5 cm) was significantly higher than that for those patients with a small tumor (< 5 cm) (p < 0.01).
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in East Asia, and its incidence in the United States is increasing (1). Although surgical resection offers a better curative option than nonsurgical treatment, approximately 70% to 80% of cases are inoperable because of associated liver cirrhosis or advanced disease at the time of presentation (2). Transcatheter arterial chemoembolization (TACE) has been widely used for the management of unresectable HCC (3, 4).
The rationale of HCC chemoembolization is based on the fact that the normal liver parenchyma receives a dual blood supply from the hepatic artery and the portal vein, but HCC is exclusively supplied by the hepatic artery (5). However, in clinical practice we frequently encounter HCCs supplied by extrahepatic collateral arteries (ExCAs) even when the hepatic artery is widely patent (6-8). Moreover, the development of ExCAs supplying a HCC prohibits effective control of the tumor by a transcatheter arterial approach such as hepatic arterial chemoembolization, hepatic arterial infusion chemotherapy, radiotherapy using radioactive materials (i.e., I-131-Lipiodol), and possibly intraarterial gene therapy. Therefore, the presence or absence of an ExCA supplying a HCC should be an essential consideration when selecting an optimal treatment method from among the currently available treatment modalities, e.g., surgical resection, percutaneous ablation using absolute ethanol or radiofrequency, or transcatheter arterial management.
However, there have been no large prospective series conducted to determine the prevalence and causative factors of ExCAs supplying HCCs. Thus, we performed this prospective study to investigate the prevalence and causative factors of ExCAs supplying HCCs at its initial presentation and during chemoembolization.
Between February 1998 and April 2000, 733 patients with newly diagnosed HCC were referred to us for TACE treatment. We excluded 254 patients from this study because of a previous history of surgical resection for HCC (n = 30), a previous history of percutaneous ethanol injection or radiofrequency ablation (n = 58), previous TACE procedures that had been performed at other institutions (n = 40), and/or they had a diffuse type HCC that the dimensions of which could not be accurately determined (n = 126). Therefore, a total of 479 patients were ultimately enrolled in this study. At the time of study commencement, our study didn't require institutional review board approval, but an informed consent was obtained from all the patients before each TACE session.
Of the 479 study subjects, there were 375 males and 104 females with ages ranging from 19 to 85 years (mean age: 57 years). A total of 1629 sessions of TACE were prospectively performed in these 479 patients (range: 1-15 sessions; mean: 3.4 sessions) until April 2004. A single TACE session was administered to 201 patients and repeated sessions of TACE were administered to 278 patients; 110 patients underwent surgical resection after one (n = 105) or two (n = 5) TACE sessions. At April 2004, 130 patients were being followed up, and 349 patients died or were lost to follow-up.
Four hundred fifty one of the 479 patients had various risk factors of HCC, namely, positivity for hepatitis B surface antigen (n = 364), antibody to hepatitis C virus (n = 52), both hepatitis B surface antigen and antibody to hepatitis C virus (n = 2), alcoholic liver cirrhosis (n = 23), or membranous obstruction of the inferior vena cava (n = 10). A diagnosis of HCC was rendered based on the results of a percutaneous needle biopsy (n = 35), surgical resection of HCC during follow-up (n = 110), or by the clinical or laboratory test results (e.g., elevated alpha-fetoprotein levels and viral markers) in combination with the typical computed tomographic (CT) and angiographic appearances and disease progression on the follow-up images (n = 334).
All patients had enhanced biphasic helical CT scans of the liver taken before the initial TACE session; the method used has been described in detail elsewhere (6-11) and it is only summarized here. The procedure was initially performed by infusing 2-12 mL of iodized oil (Lipiodol; Andre Gurbet, Aulnay-sous-Bois, France) and 10-60 mg of doxorubicin hydrochloride emulsion (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea) until arterial flow stasis was achieved and/or iodized oil appeared in the portal branches. If the initial hepatic arterial blockade was insufficient because of a large sized mass or arterioportal shunting, then embolization was performed with absorbable gelatin sponge particles (1-2 mm in diameter; Gelfoam; Upjohn, Kalamazoo, MI) soaked in a mixture of 4-6 mg of crystalline mitomycin (Mitomycin-C; Kyowa Hakko Kogyo, Tokyo, Japan) and 10 mL of nonionic contrast medium.
Unenhanced CT scanning of the entire liver was performed 1-3 weeks after TACE to assess the liver for traces of iodized oil, and enhanced biphasic helical CT was performed 1-2 weeks before the next TACE session to assess the efficacy of the previous treatment and to detect the residual viable tumor or any recurrent tumor. Although the normal period between the TACE sessions was 3 months, these repeat sessions were tailored to the patients' tolerance to the procedure and the tumor response.
One of two experienced interventional radiologists (J.W.C and J.H.P) reviewed the initial and/or follow-up CT scans, angiograms and α-fetoprotein serum levels, and they determined whether a primary or recurrent tumor was possibly being supplied by ExCAs. A primary tumor was defined as the tumor initially present, and a recurrent tumor was defined as a distinct tumor that newly appeared during the follow-up period after TACE.
The findings that suggested an ExCA supplying a tumor were a subcapsular location or exophytic tumor growth, a peripheral iodized-oil retention defect within the tumor or a peripherally located portion of viable tumor on a follow-up CT scan, and hypertrophied ExCAs around the tumor on CT scan, a persistent elevation of serum α-fetoprotein level even after successful chemoembolization via the hepatic arteries, and the presence of a peripheral tumor staining defect according to hepatic arteriography (6-8, 12-16). In the patients with these suggestive findings, we localized the ExCAs by the tumor location and we performed selective angiography on all suspected collaterals capable of supplying the tumor, i.e., the inferior phrenic, omental, cystic, adrenal, intercostal, gastric, internal mammary, superior mesenteric, renal and renal capsular arteries (6-8, 12-16). When typical tumor staining was observed by selective angiography of a suspected vessel, we concluded that the tumor was supplied by an ExCA (Figs. 1, 2, 3).
When a tumor was located in the bare area of the liver, we performed selective angiography on the right inferior phrenic artery and/or the right adrenal artery; when a tumor was located in the superoanterior portion of the liver, we performed selective angiography of the internal mammary artery; when tumor was in contact with the right kidney, we performed selective angiography of the right renal artery and right adrenal artery; when tumor was in contact with omental fat with an exophytic growth pattern, we performed selective angiography of the gastroduodenal artery and of the right and left gastroepiploic arteries to find the omental branches supplying the tumor; when a tumor invaded or was in contact with the right lateral thoracic wall, we performed selective angiography of the lower intercostal arteries; when an exophytic tumor was located in the left lateral segment of the liver, we performed selective angiography of gastric arteries; and when an exophytic tumor was in contact with the colon, we performed selective angiography of the colic branch of the superior mesenteric artery.
If no ExCA was found at an initial TACE session, then the iodized oil distribution in the tumor on the unenhanced CT performed within three weeks after the initial session became important for determining the presence or absence of an ExCA supplying the tumor at its initial presentation. When a peripheral iodized oil retention defect in tumor was demonstrated by an unenhanced CT scan and an ExCA supplying the tumor defect area was found at the 2nd TACE session, we considered that an ExCA was present at the initial session (7).
We evaluated the following potential causative factors for the development of ExCAs; the size and location of the tumor, and the patency of the hepatic artery. Tumors were assigned to liver segments in accord with Couinaud classification (17). If a tumor occupied two or more segments, then its location was assigned to the dominant segment. In cases of multinodular tumors, only the largest was analyzed. Tumor size was defined as the largest tumor diameter on the transverse CT scans.
The tumor locations were also classified as surface or non-surface. If a tumor showed exophytic growth or it was observed to reach the liver margin on CT, then it was considered to have a surface location, but if it did not reach the liver margin, then it was considered to have a non-surface location. For the subgroups of tumor surface location, we also tested the hypothesis that a tumor located in a bare area may have a higher prevalence of ExCAs. Because the bare area of a liver cannot be clearly outlined, our operational definition of the bare area of the liver was the posterior surface of hepatic segment 7 and the posterior half of the diaphragmatic surface of hepatic segment 8. If a tumor in the subgroup with a surface location contacted the bare area, then it was classified as being located in the bare area, and if not, it was considered to be in the non-bare area.
Occlusion of the hepatic artery that was caused by repeated TACE sessions was defined as the obliteration of the segmental hepatic arterial branches with a lack of arterial perfusion in the corresponding liver portion. Celiac axis occlusion was also recorded when a superior mesenteric angiogram showed opacification of all the celiac axis branches.
Univariate analyses of the correlation of ExCA formation with several variables (gender, celiac axis occlusion, and a surface or bare area tumor location) were performed by using Fisher's exact test for nominal variables and the t-test for continuous variables (tumor size and patient age). Variables with a p value of < 0.25 according to univariate analysis were chosen as the variables for multiple logistic regression analysis. For the subgroups of a surface location, univariate and multivariate analyses were also performed in the same manner, and univariate analysis was also performed after stratification by tumor size.
The cumulative probability curves of ExCA formation were drafted using the Kaplan-Meier method and the results were compared using the log-rank test. Patients lost to follow-up were censored at the date of last observation. Patients who underwent surgical resection for HCC after TACE were censored at the date of the operation. We did not include data for repeated TACE sessions due to recurred tumor after surgery in the analysis. P values < 0.05 were considered to indicate statistical significance. Data processing and analysis were performed using SPSS version 10.0 (SPSS, Chicago, IL).
In 82 (17%) of the 479 patients, 108 ExCAs were found to supply HCCs at the initial TACE session (Table 1). The numbers of the initial ExCAs per patient were one (n = 61), two (n = 17), three (n = 3), or four (n = 1). Although celiac occlusion was present in nine (1.9%) patients, the hepatic artery was widely patent at the initial TACE session in all the patients. The tumor sizes and locations are summarized in Table 2. Univariate analysis showed that tumor size (p < 0.01), patient age (p = 0.02), a surface location (p < 0.01), and a bare area location (p < 0.01) were significantly associated with the presence of ExCAs. Multiple logistic regression analysis showed that only tumor size was predictive of ExCA formation (p < 0.01, odds ratio = 1.737, confidence interval: 1.533 to 1.969). As the tumor size increased, the probability of ExCAs formation was also increased (Fig. 4).
In the subgroup of the 349 patients who had tumors with a surface location, univariate analysis showed that tumor size (p < 0.01) and a bare area location (p < 0.01) were significantly associated with the presence of ExCAs. Multiple logistic regression analysis showed that tumor size was predictive of ExCA formation (p < 0.01, odds ratio = 1.735, confidence interval; 1.532 to 1.966). After stratification by tumor size, a bare area location was not associated with the presence of ExCAs for tumors less than 10 cm in diameter (Table 3).
During repeated TACE sessions for 278 patients, 97 ExCAs were detected at the 2nd to the 15th (mean±SD: 5.5±2.7) TACE sessions in 70 (25%) patients (Table 1). Four of these 70 patients had also ExCAs observed at the initial TACE session. Therefore, 148 (31%) of the 479 study subjects had ExCAs at the initial or repeat TACE sessions. In 35 of the above 70 patients, ExCAs supplied the primary tumors that were present at the initial TACE session. In the other 35 patients, ExCAs supplied recurrent tumors. Occlusion of the segmental hepatic artery was detected in 16 patients during repeated sessions of TACE. In 12 of these 16 patients, ExCAs were detected at the 4th-11th TACE sessions.
The cumulative probability curve for the presence of ExCAs is shown in Figure 5. As the number of TACE sessions increased, the cumulative probability of ExCAs also increased, and particularly for those ExCAs supplying recurrent tumors. The cumulative probability of ExCAs in patients with a large tumor (≥ 5 cm) was significantly higher than that for those patients with a small tumor (< 5 cm) (p < 0.01). Whereas patients who initially had a large primary tumor usually had ExCAs supplying the primary tumor, patients with a small tumor usually had ExCAs supplying a recurrent tumor after several TACE sessions.
For the various strategies to treat hepatic tumors by the transcatheter arterial approach, knowledge about the blood supply of hepatic tumors is essential not only for understanding the strategies' limitations but also to develop an adequate protocol to improve their therapeutic efficacy.
This study describes the prevalence and causes of ExCAs that supply HCCs. At the initial presentation, 17% of all tumors and 23% of the tumors in the subcapsular location or that demonstrated exophytic growth had ExCAs.
In the past literature, most ExCAs were discovered after the hepatic arterial blood supply had been interrupted by surgical ligation, repeated embolization or mechanical injury to the hepatic artery (18-20). However, we found that almost all the patients with ExCAs had widely patent hepatic arteries. Among the study population, only nine patients displayed celiac axis occlusion with patent peripheral hepatic arteries. Therefore, hepatic artery occlusion is not the major cause of developing ExCAs.
In this study, in line with the fact that a surface tumor location is a prerequisite for the formation of ExCAs, the most important factor associated with ExCA formation was the tumor size rather than interruption of the hepatic artery. Table 2 and Figure 4 clearly show the abrupt increase in the prevalence of an extrahepatic collateral supply to HCC with a tumor size in the range of 4-6 cm. When the tumor was smaller than 4 cm, the prevalence of ExCA at the initial TACE secession was less than 3% (6 of 253 tumors). Instead, when the tumor was bigger than 6 cm, the prevalence of ExCAs was 63% (65 of 107 tumors).
As the number of TACE sessions increased, the cumulative probability of ExCAs being present also increased (Fig. 5). For patients with large primary tumors (≥ 5 cm), most ExCAs supplied the primary tumor. The increasing probability of finding ExCAs for large tumors during repeated TACE procedures can be explained by the regrowth of invisible tiny tumor foci that were initially supplied by ExCAs or there was local tumor progression (i.e., the primary tumor grows to reach a subcapsular location or it grows exophytically, or it invades an adjacent organ, resulting in the creation of ExCAs). In contrast, for the patients with small primary tumors (< 5 cm), most of the ExCAs supplied the recurrent tumors that developed after multiple TACE sessions. Peripheral hepatic artery attenuation or occlusion was frequently associated with these circumstances. Therefore, the increasing probability of ExCAs for small tumors during repeated TACE procedures can be explained by a sequence of events: peripheral hepatic artery occlusion due to repeated TACE procedure, the development of ExCAs supplying the peripheral zone of the liver parenchyma, and then subsequent remote site tumor recurrence at the peripheral zone supplied by the ExCAs.
There is a close contact between the liver and the diaphragm, and the blood supply to the diaphragm can reach the liver by direct adherence. Thus, the right inferior phrenic artery is the most common collateral pathway (18). In our study, the right inferior phrenic artery accounted for half of all the ExCAs.
It is believed to be likely that ExCAs develop early at the bare area of the liver because the diaphragm and the liver are in direct contact without any capsular barrier (18). We tested the hypothesis that a tumor located in a bare area may have a higher prevalence of ExCAs. On the univariate analysis, a tumor located in a bare area had more ExCAs than did the tumors in a non-bare area. However, multivariate analysis showed that only tumor size was associated with ExCA formation. After stratification by tumor size, a bare area location was not associated with the presence of ExCAs for the tumors with a surface location and also for tumors less than 10 cm in diameter. This result may be caused by the dominating factor of tumor size.
Development of ExCAs in HCC is certainly a situation that limits the efficacy of TACE. Chemoembolization through ExCAs can be attempted to improve the therapeutic efficacy of TACE. In this situation, special care should be taken to monitor for ischemic injury to and chemotoxicity in the organs supplied by ExCAs, and selective catheterization should be accomplished by placing the catheter tip as close as possible to a specific branch or branches supplying the neoplasm. Therefore, not every detected ExCA can be embolized because of failure that is incurred with performing superselective catheterization or because of the various TACE-associated complications (8, 9, 21, 22). Acute gastroduodenal mucosal lesions or ulcerations have been described as common TACE complications (21). When the cystic artery is occluded during TACE, then gallbladder infarction or cholecystitis can occur (9). Spinal cord injury has been reported after intercostal arterial embolization (9).
There are several retrospective studies that TACE through ExCAs was useful for preventing tumor progression (7, 13-16). However, it is not possible to draw any conclusions as to whether TACE administered in this manner prolongs the patient's life-span. Therefore, further studies are warranted to investigate the survival benefits of TACE via ExCAs for treating HCC.
Some limitations of the present study should be mentioned. First, we performed selective angiography of the ExCAs in patients whom we suspected of having a blood supply to a tumor. We probably missed unsuspected ExCAs in some patients. Moreover, in clinical practice, selective angiography was not performed in some patients who were at an advanced stage of disease despite our suspicion about a collateral blood supply. Thus, the prevalence of ExCAs in this study was probably underestimated. Second, although we showed that the cumulative probability of ExCAs increased during repeated TACE, we did not statistically reveal that repeated TACE itself was a causative factor for the development of ExCAs. Third, although the attenuation of the hepatic artery is somewhat subjective to readers, we did not take the attenuation of the hepatic artery into account when determining the causative factors of ExCAs. Instead, we took account of only the occlusion of the segmental hepatic artery, which was detected in 16 patients during repeated sessions of TACE.
In conclusion, the presence of ExCAs supplying HCC is rather common, and the size of the tumor is a significant causative factor for the development of these collateral arteries.
Figures and Tables
Fig. 1
A 51-year-old man with hepatocellular carcinoma.
A. Enhanced CT scan shows enhancing nodular lesion (arrowhead) 2 cm in size in the liver segment 7.
B. Right hepatic angiogram shows staining of a small nodular tumor (arrow).
C. Right inferior phrenic angiogram shows staining of small nodular tumor (arrowheads) that are supplied by the right inferior phrenic artery. Note the staining of the adrenal gland (arrow) that's supplied by the superior adrenal artery (open arrow) from the right inferior phrenic artery.
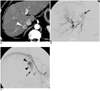
Fig. 2
A 39-year-old man with hepatocellular carcinoma.
A. Enhanced CT scan shows a large exophytic mass (arrowheads) in the right liver lobe with prominent vessels (arrows) around the tumor.
B. Celiac angiogram shows a large stained tumor (arrowheads) that's supplied by the right hepatic artery and omental branches (arrow) from the gastroepiploic artery. Note the defect (dotted circle) of tumor staining.
C. Right inferior phrenic angiogram shows tumor staining (arrowheads) corresponding to the defect on the celiac angiogram.
D. The superior mesenteric angiogram shows that hypertrophied colic branch (arrow) supply the tumor. Omental branches (arrowheads) supplying the tumor are also opacified via the pancreaticoduodenal arcade.
E. Gastroepiploic angiogram shows multiple hypertrophied omental branches (arrowheads) supplying the tumor. Note the tip of the microcatheter (arrow).
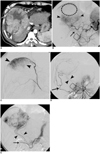
Fig. 3
A 60-year-old man with hepatocellular carcinoma.
A. Enhanced CT scan after six sessions of chemoembolization shows small enhancing tumor (arrowheads) in the liver segment 6. Note the nodule that has taken up iodized oil (arrow) without viable tumor.
B. Celiac angiogram at the seventh session of chemoembolization shows a small stained tumor (arrow) that's supplied by an omental branch (arrowhead) from the gastroduodenal artery. Note attenuation of the hepatic artery (curved arrow) caused by repeated chemoembolization.

Fig. 4
The relationship of the tumor size and the presence of extrahepatic collateral arteries supplying hepatocellular carcinoma at the initial transcatheter arterial chemoembolization.

Fig. 5
Cumulative probability curve of the extrahepatic collateral supply.
A. Kaplan-Meier plot shows the cumulative probability curve of extrahepatic collateral arteries supplying all tumors (A) and the primary tumor only (B).The gap between the two curves means the probability of extrahepatic collateral arteries for recurrent tumors during repeated TACE sessions.
B. The Kaplan-Meier plot shows the cumulative probability curve of the extrahepatic collateral arteries in patients who all had large tumors (≥ 5 cm) (A), primary large tumors (B), all small tumors (< 5 cm) (C), and primary small tumors (D). The cumulative probability of ExCAs was statistically different between large tumor (≥ 5 cm, A) and small tumor (< 5 cm, C) (log-rank test, p < 0.01). The gap between curves A and B stands for the probability of extrahepatic collateral arteries for recurrent tumors in patients with large tumors (≥ 5 cm). The gap between curves C and D represents the probability of extrahepatic collateral arteries for recurrent tumors in patients with small tumors (< 5 cm).

References
1. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999. 340:745–750.
2. Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004. 127:S218–S224.
3. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002. 35:1164–1171.
4. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003. 37:429–442.
5. Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, et al. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983. 52:2193–2200.
6. Kim JH, Chung JW, Han JK, Park JH, Choi BI, Han MC. Transcatheter arterial embolization of the internal mammary artery in hepatocellular carcinoma. J Vasc Interv Radiol. 1995. 6:71–74.
7. Chung JW, Park JH, Han JK, Choi BI, Kim TK, Han MC. Transcatheter oily chemoembolization of the inferior phrenic artery in hepatocellular carcinoma: the safety and potential therapeutic role. J Vasc Interv Radiol. 1998. 9:495–500.
8. Kim HC, Chung JW, Lee W, Jae HJ, Park JH. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005. 25:S25–S39.
9. Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996. 198:33–40.
10. Kwon JW, Chung JW, Song SY, Lim HG, Myung JS, Choi YH, et al. Transcatheter arterial chemoembolization for hepatocellular carcinomas in patients with celiac axis occlusion. J Vasc Interv Radiol. 2002. 13:689–694.
11. Song SY, Chung JW, Kwon JW, Joh JH, Shin SJ, Kim HB, et al. Collateral pathways in patients with celiac axis stenosis: angiographic-spiral CT correlation. Radiographics. 2002. 22:881–893.
12. Nakai M, Sato M, Kawai N, Minamiguchi H, Masuda M, Tanihata H, et al. Hepatocellular carcinoma: involvement of the internal mammary artery. Radiology. 2001. 219:147–152.
13. Miyayama S, Matsui O, Akakura Y, Yamamoto T, Nishida H, Yoneda K, et al. Hepatocellular carcinoma with blood supply from omental branches: treatment with transcatheter arterial embolization. J Vasc Interv Radiol. 2001. 12:1285–1290.
14. Won JY, Lee DY, Lee JT, Park SI, Kim MJ, Yoo HS, et al. Supplemental transcatheter arterial chemoembolization through a collateral omental artery: treatment for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2003. 26:136–140.
15. Park SI, Lee DY, Won JY, Lee JT. Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J Vasc Interv Radiol. 2003. 14:461–468.
16. Miyayama S, Matsui O, Nishida H, Yamamori S, Minami T, Shinmura R, et al. Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol. 2003. 14:1155–1161.
17. Couinaud C. Le foie: études anatomiques et chirurgicales. 1957. Paris, France: Masson;9–12.
18. Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T. Angiographic classification of hepatic arterial collaterals. Radiology. 1982. 144:485–494.
19. Michels NA. Collateral arterial pathways to the liver after ligation of the hepatic artery and removal of the celiac axis. Cancer. 1953. 6:708–724.
20. Doppman JL, Girton M, Kahn R. Proximal versus peripheral hepatic artery embolization experimental study in monkeys. Radiology. 1978. 128:577–588.
21. Hirakawa M, Iida M, Aoyagi K, Matsui T, Akagi K, Fujishima M. Gastroduodenal lesions after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Am J Gastroenterol. 1988. 83:837–840.
22. Arora R, Soulen MC, Haskal ZJ. Cutaneous complications of hepatic chemoembolization via extrahepatic collaterals. J Vasc Interv Radiol. 1999. 10:1351–1356.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download