Abstract
Objective
Hyperdense lesions can frequently be observed on the CT obtained immediately after intra-arterial (IA) thrombolysis, and it is sometimes difficult to differentiate contrast extravasation from the hemorrhagic lesions. The purposes of this study are to classify the hyperdense lesions according to their morphologic features and to track the outcome of those lesions.
Materials and Methods
Among the 94 patients who suffered with anterior circulation ischemic stroke and who were treated with IA thrombolysis, 31 patients revealed hyperdense lesions on the CT obtained immediately after the procedure. The lesions were categorized into four types according to their volume, shape, location and density: cortical high density (HD), soft HD, metallic HD and diffuse HD. The follow-up images were obtained 3-5 days later in order to visualize the morphologic changes and hemorrhagic transformation of the lesions.
Results
Among the 31 patients with HD lesions, 18 (58%) showed hemorrhagic transformation of their lesion, and six of them were significant. All the cortical HD lesions (n = 4) revealed spontaneous resolution. Seven of the soft HD lesions (n = 13) showed spontaneous resolution, while the rest of the group showed hemorrhagic transformation. Among them the hemorrhage was significant in only two patients (2/6) who did not achieve successful recanalization. All the metallic HD lesions (n = 10) resulted in hemorrhagic transformation; among them, three cases (30%) with a maximum CT value more than 150 HU (Hounsfield unit) subsequently showed significant hemorrhagic transformation on the follow-up CT. There were four diffuse HD lesions, and two of them showed hemorrhagic transformation.
Conclusion
The parenchymal hyperdense lesions observed on the CT obtained immediately after IA thrombolysis in ischemic stroke patients exhibited varying features and they were not always hemorrhagic. Most of the soft HD lesions were benign, and although all of the metallic HD lesions were hemorrhagic, some of them were ultimately found to be benign.
Immediately after completing intra-arterial (IA) thrombolysis, a non-contrast CT (NCCT) scan is usually performed to check for the possible occurrence of hemorrhage, as well as for progression of the initial ischemic lesion (1, 2). At that time, it's not uncommon to note parenchymal high-density lesions on the CT whether or not the procedure was successful (3-5). These hyperdense lesions have been documented and described since 1993 as being either hemorrhage or the result of extravasation of the contrast media that's used during the angiography and intravascular interventional procedures in either the anterior or posterior circulation territories (5, 6). It is not easy to differentiate any extravasation of contrast material from hemorrhagic lesions according to this high density (HD) phenomenon (7).
It's been suggested by recent studies that contrast extravasation is highly associated with the formation of parenchymal hematoma; therefore, this should be considered as a negative prognostic sign for the procedure (3, 8). In addition, the presence of these hyperdense areas was noted to be a significant risk factor for severe hemorrhagic transformation (9). In contrast to a recent report by Yoon et al. (8), in our experience, the hyperdense lesions have not always been hemorrhagic, and we have frequently experienced spontaneous resolution of the hyperdense lesions.
The purposes of this study are to classify the HD lesions according to their morphologic features as noted on the NCCT obtained immediately after IA thrombolysis, and to analyze the fate of those hyperdense lesions on the follow-up imaging in terms of their spontaneous resolution and hemorrhagic transformation.
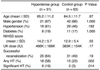
Between January 1999 and June 2004, IA thrombolysis was performed in 94 consecutive patients who suffered with acute ischemic stroke in the carotid artery territory. On retrospective review of the NCCT obtained immediately after the procedure, 31 of the 94 study patients showed varying patterns of hyperdense lesions that seemed to appear after the procedures. For the convenience of the analysis, we divided the study patients into two groups, i.e., the hyperdense group and the control group. The latter group represents those patients without hyperdense lesions on the post-procedure NCCT. The initial severity of the patients' symptoms was evaluated using the National Institutes of Health Stroke Scale (NIHSS) score, which was recorded before the procedure. The presence of hypertension and/or diabetes was also noted. The basic clinical data are summarized in Table 1.
All the procedures were undertaken if they could be initiated within six hours of the initial onset of symptoms. We performed image-based patient selection using CT and/or multimodal MRI, including the diffusion-weighted imaging (DWI), perfusion studies, MR angiography, gradient-echo imaging (GRE) and T2-weighted imaging (T2-WI). We used the clinical and CT inclusion/exclusion criteria of PROACT II (1). For the MR scans, the only inclusion criterion was the presence of a significant diffusion/perfusion mismatch. We did not conduct the procedure if there was any hemorrhage, if the extent of the DWI high signal lesion exceeded more than half of the involved middle cerebral artery (MCA) territory or if there was no visible arterial steno-occlusive lesion proximal to the M2 portion of the MCA on the MR angiography. An informed consent for the thrombolytic procedure was obtained from the patients' next of kin.
The recanalization procedure was performed with the coaxial microcatheter system and using a basically identical methodology as was applied in the PROACT II study (1). The thrombolytic agent used was urokinase (10,000-20,000 IU/min, Green Cross Pharm., Korea). If the patient presented within three hours after the initial onset of symptoms, tissue-plasminogen activator (0.6 mg/kg, Actylase, Berlinger-Ingelheim, Germany) was administered intravenously before the procedure. Mechanical clot disruption using micro-guidewires, snare devices and/or angioplasty balloon catheters was attempted in some patients in order to facilitate the effect of the thrombolytics. The use of systemic heparin was prohibited during the procedure and for 24 hours thereafter.
The procedure was terminated when recanalization was achieved (Thrombolysis In Myocardial Infarction [TIMI] of grade ≥ 2) or when the maximum permissible dose of urokinase had been used (1,000,000 IU).
Non-contrast CT scan was performed immediately after the procedure to check for the occurrence of hemorrhagic change before the patient's admission to the neurological intensive care unit. Every patient with hyperdense lesions on the post-procedure NCCT was followed up with a CT scan 24 hours after performing the IA thrombolysis. The follow-up MR imaging, including DWI, T2-WI and GRE, was performed three to five days after the procedure unless there was a suspicion of rapid progression of the infarction or any hemorrhagic complication, which was then immediately monitored by CT.
The findings of the pre- and post-procedure angiographies, the post-procedure NCCT and follow-up CT/MR images were analyzed by a board-certified neuroradiologist (D.H.L.) and a third-year radiology resident (Y.M.J.) with focusing on the anatomic site and the degree of the initial arterial occlusion, the final TIMI grade after thrombolysis, the presence of a hyperdense lesion on NCCT and the occurrence of hemorrhagic transformation on follow-up.
We analyzed the morphologic characteristics of the hyperdense lesions noted on the post-procedure NCCT. The lesion location was categorized as cortical (Fig. 1A) or non-cortical (Figs. 1B-D). The CT Hounsfield unit (HU) and the total volume were measured using a built-in calculation tool in the hospital's picture archiving and communication system (PACS, Petavision; Seoul, Korea). A rectangular region-of-interest (ROI: 25 mm2 in area) was placed at the highest density area in the lesion to measure the CT unit. In addition, the largest area of the lesion was measured on the most representative slice.
On retrospectively reviewing the findings of the hyperdense lesions observed on NCCT, it was possible to categorize them into four types according to their location in the brain parenchyma and their CT density, volume and shape. Cortical HD was defined as a hyperdense lesion that was limited to the cortices (Fig. 1A), soft HD was defined as a hyperdense lesion with a maximum CT unit measurement < 80 HU and a small volume without contour bulging (Fig. 1B), metallic HD was defined as a hyperdense lesion with a maximum CT unit measurement > 80 HU (Fig. 1C) and a larger volume with bulging contour, and diffuse HD was defined as a hyperdense area involving a large vascular territory (Fig. 1D).
Hemorrhagic transformation of the HD lesion observed on NCCT was defined as any area of persistent HD on the follow-up CT or any dark signals within the pre-existing HD area on the GRE MR images obtained at least 24 hours after the procedure. Significant hemorrhage was defined as a confluent hematoma with a significant mass effect that compressed and displaced the surrounding brain parenchyma.
All of the analysis was performed by the two interpreters working in consensus. This retrospective study was approved by the local institutional review board of our hospital (AMC IRB No. 2005-0021).
The statistical analyses were performed using a statistical software package (SPSS for Windows, version 11.0; Chicago, IL). To determine the differences between the HD group and the control group, univariate analyses were performed using Fisher's exact test for the categorical variables, i.e., gender, hypertension, diabetes, the arterial occlusion site, success with recanalization, hemorrhagic transformation and significant hemorrhage, and the Mann-Whitney U test was used for the continuous variables, i.e., age, NIHSS score and the total dose of urokinase. Fisher's exact tests were then performed again to determine the possible influencing factors, i.e., the success of recanalization, the NIHSS score and the CT unit of the lesions, the hemorrhagic transformation and significant hemorrhage of the soft and metallic high-density lesions. A threshold of 15 and 150 HU was applied to dichotomize the NIHSS scores and the CT unit of the lesions, respectively. We omitted any statistical test for the cortical and diffuse HD lesions because of the small number of such cases. A value of p < .05 was considered to be significant.
The baseline characteristics of each group are shown in Table 1. The mean age of all 94 patients treated with IA thrombolysis was 64.7±11.5 (mean±SD). The median NIHSS score at admission was 14 (range: 0 to 22). Angiography performed immediately before the IA thrombolysis showed occlusion of the ICA in 25 patients, occlusion of the carotid T portion in 16 patients, occlusion of the proximal M1 segments of the MCA in 28 patients and occlusion of the distal M1 segment of the MCA in 25 patients. Of the 94 patients who underwent IA thrombolysis, 31 (33%) were placed in the hyperdense group and 63 (67%) were placed in the control group.
Of all the 94 patients, any amount of hemorrhagic transformation was observed in 33 patients (35%). Fifteen (25%) of the 63 control-group patients and 18 (58%) of the 31 hyperdense-group patients became hemorrhagic; this difference was statistically significant (p = 0.002). Significant hemorrhage was observed in eight (24%) of the 33 hemorrhagic patients. The overall rate of significant hemorrhage was 8.5% (8 patients); two (3.5%) were in the control group and six (19.4%) were in the hyperdense group. The difference was statistically significant (p = 0.014).
According to our morphologic criteria, the 31 hyperdense lesions were further categorized into cortical HD in four patients (13%), soft HD in 13 patients (42%), metallic HD in 10 patients (32%) and diffuse HD in four patients (13%).
In all groups, there was no significant difference in the clinical factors (age, gender, hypertension, diabetes, the site of arterial occlusion, the total dose of urokinase and the CT findings including volume and the highest number of CT unit of the hyperdense lesions) between the patients with hemorrhagic transformation and the patients without hemorrhagic transformation. The mean volume and CT unit of each group are summarized in Table 2. When the number of patients falling into the tentative intervals of the CT density was plotted as a graph, an obvious bimodal frequency distribution was observed; this showed a gap between 70 HU and 89 HU (Fig. 2).
All four of the cortical HD lesions had resolved spontaneously on the follow-up CT scans obtained 24 hours after the procedure (Fig. 3).
Of all the 13 soft HD lesions, the hyperdense lesions disappeared on the follow-up CTs in seven patients (Figs. 4A, B). Eight of the 13 patients underwent successful recanalization. Hemorrhagic transformation within the infarct area occurred in six patients. Significant hemorrhagic transformation was observed in the two patients who experienced failure on their IA thrombolysis procedures (Figs. 4C, D). There was a tendency for significant hemorrhage in the patients who experienced failure on their IA thrombolysis, although the difference was statistically insignificant (none of 8 [0%] versus 2 of 5 [40%], respectively, p = 0.128).
All 10 patients with metallic HD became hemorrhagic; there was no correlation between the success of recanalization and the hemorrhagic transformation in this group. Among them, three cases (30%) showed subsequent significant hemorrhagic transformation on the follow-up CT. None of the four patients who had an initial NIHSS score less than 15 showed significant hemorrhage (Figs. 5A, B). Further, none of the six patients who had a maximum of less than 150 CT unit showed significant hemorrhage. In contrast, three (75%) of the four patients who had a maximum of 150 or more CT unit showed significant hemorrhage (Figs. 5C, D). The difference between these two groups was significant (0% versus 75%, respectively, p = 0.033).
All four patients with diffuse HD experienced successful recanalization. The CT unit of all of the lesions were less than 80 HU. Two patients (2/4, 50%) having volumes of hyperdense lesion greater than 30 mL experienced hemorrhagic transformation. Of these two patients, the patient with the largest volume (89 mL) showed significant hemorrhage.
The causative mechanism of those CT high densities that are observed after performing IA thrombolysis could be simply speculated as the result of extravascular leakage (extravasation) of contrast media that was given during the angiography and interventional procedure (4, 7, 9). The major premise for explaining that extravasation is disruption of the blood-brain barrier (BBB) (10-12). Some of the lesions might be caused by the erroneous injection of contrast media into the parenchyma through small arterial side branches, such as the lenticulostriate arteries. The pattern of those high densities would vary according to the degree of BBB disruption and the location of the lesions (3, 10).
The incidence of hyperdense lesions, in our study, on NCCT after performing IA thrombolysis was 33%, the hemorrhage rate of those lesions was 55% and the rate of significant hemorrhage was 33%, and all these rates were comparable to those of the other previous studies (3, 8, 9). We could categorize the hyperdense lesions into four types according to the morphologic features of these lesions, i.e., cortical HD, soft HD, metallic HD and diffuse HD. This scheme was very helpful for predicting the development of hemorrhagic transformation in those lesions, as we could see by the result of this study; the clinical and radiological behaviors of the hyperdense lesions varied according to the type of the lesion.
All four patients with cortical HD lesions showed disappearance of those lesions on the follow-up CT that was taken 24 hours after IA thrombolysis. It was not uncommon to see corresponding small cortical lesions upon conducting a retrospective review of the preprocedure CT or MR findings of those patients. We believe that those cortical HD lesions may have been caused by staining of the contrast media that was injected during the procedure at the already-infarcted cortex, which was neglected during the initial evaluation before performing IA thrombolysis.
It is notable that a significant number of cases of soft HD lesions show spontaneous resolution of the HD on the follow-up CT scans, the same as Wildenhain et al. have already mentioned (4). However, there was a tendency toward incurring significant hemorrhage in the patients whose MCA was not successfully recanalized by the thrombolytic procedure. Delayed recanalization of the remaining vascular occlusion might provoke the development of delayed hemorrhage in those patients. Persistent occlusion might result in progressive tissue infarction, and this tissue could be more prone to reperfusion injury by the subsequent recanalization of the refractory lesion (12).
The metallic HD lesion is nearly identical to the lesion described as 'contrast extravasation', as was coined by Yoon et al. (8) to describe the hyperdense lesion that had a maximum CT unit value greater 90 HU and it was persistent on the follow-up CT scans obtained at least 24 hours after the procedure. When the CT units of all 31 hyperdense lesions in our series were plotted, we observed a bimodal pattern of lesion distribution (Fig. 2). There was no lesion showing a CT density between 70 HUs and 89 HU. Therefore, a threshold of 80 HU could be applied in our cases to discriminate between the soft HD lesions and the metallic HD lesions.
It is notable that a large proportion (70%) of the metallic HD lesions did not show 'significant' hemorrhagic transformation, although all the lesions in our series became hemorrhagic in some degree. This observation is far different from that of Yoon et al. They reported 100% of symptomatic hemorrhage in case of contrast extravasation in their study (8).
The CT units and NIHSS score emerged as prognostic factors in case of metallic HD. Mericle et al. (5) have already suggested that any hyperdensity greater than 150 HU was a poor prognostic factor. A similar result was noted in our study, and significant hemorrhage was more frequent for the metallic HD with a CT value more than 150 HU. We also observed that none of the four patients with an initial NIHSS score less than 15 showed significant hemorrhages for the cases of metallic HD.
In our series, regardless of the CT density or the location of hyperdense lesions, an extensive hyperdense lesion spreading over most of a large vessel territory infarct area was classified as another subgroup, i.e., the diffuse HD group, because the extent or volume of the hyperdense lesion could also be considered as a prognostic factor (5). In our present study, all the patients with the largest volume experienced significant hemorrhage.
The term "symptomatic hemorrhage secondary to ischemic stroke" implies a clear causal relationship between clinical deterioration and the hemorrhagic transformation, and this has been defined from both the clinical and radiological viewpoints (13). In our experience, it was not easy to define a hemorrhagic lesion as symptomatic on a clinical basis. Therefore, we used hemorrhagic transformation with a significant mass effect as a surrogate indicator for significant hemorrhagic transformation. If we followed the European Cooperative Acute Stroke Study (ECASS) classification of hemorrhagic transformation (14), the parenchymal hematoma type 2 (PH2) and part of the hemorrhagic infarction type 2 (HI2) with a mass effect could be included in this category. There was a PH2 pattern of hemorrhage in our six cases of significant hemorrhagic transformation in the deep subcortical area, except for one case of HI2 that occurred in a patient with diffuse HD.
As a result of our analyses, the study results can be applied to the management of post-IA thrombolysis patients to better predict and manage their hemorrhagic transformations. The possibility of subsequent hemorrhagic transformation is higher for the cases with soft HD lesions and failed recanalization and also for the cases with metallic HD lesions. Strict control of blood pressure and abstinence from using antithrombolytics are recommended for such patients (15). Using neuroprotectants, and especially those drugs having a mechanism of BBB stability, could be helpful in those cases (16, 17).
Our study's small sample size for the statistical analysis and the retrospective study design hinders us from drawing any conclusive answers. Other limitations of the current study are the image-based estimation of the significant hemorrhagic transformation, which was due to the lack of clinical data on symptomatic hemorrhage, and the study lacked a functional outcome analysis. In conclusion, the parenchymal high densities observed on NCCT immediately after IA thrombolysis did not always become hemorrhagic. Most of the soft HD lesions had a benign prognosis, and especially in the patients who achieved successful recanalization of the occluded artery. Care must be taken in case of soft HD lesions with failed recanalization and most of the metallic HD lesions, although some of these metallic HD lesions were ultimately benign.
Figures and Tables
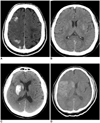 | Fig. 1Examples of the four types of hyperdense lesions on the non-contrast CT obtained immediately after intra-arterial thrombolysis. |
 | Fig. 2A histogram showing the frequency of patients with the tentative intervals of CT density as the χ axis. A bimodal frequency distribution is obvious. There is a gap between 70 HU and 89 HU. |
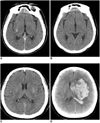 | Fig. 4A 53-year-old man (A, B) and a 63-year-old man (C, D) show the typical 'soft high density' lesions with different outcomes. |
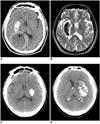 | Fig. 5A 58-year-old man (A, B) and a 67-year-old man (C, D) show typical 'metallic high density' lesions with different outcomes. |
References
1. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. Jama. 1999. 282:2003–2011.
2. Higashida R, Furlan A, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol. 2003. 14:S493–S494.
3. Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: incidence and clinical significance. Stroke. 2001. 32:2042–2048.
4. Wildenhain SL, Jungreis CA, Barr J, Mathis J, Wechsler L, Horton JA. CT after intracranial intraarterial thrombolysis for acute stroke. AJNR Am J Neuroradiol. 1994. 15:487–492.
5. Mericle RA, Lopes DK, Fronckowiak MD, Wakhloo AK, Guterman LR, Hopkins LN. A grading scale to predict outcomes after intra-arterial thrombolysis for stroke complicated by contrast extravasation. Neurosurgery. 2000. 46:1307–1314. discussion 1314-1315.
6. Yoon W, Seo JJ, Kang HK. Bilateral paramedian thalamic contrast enhancement on CT after Intra-arterial thrombolysis. Korean J Radiol. 2005. 6:41–43.
7. Komiyama M, Nishijima Y, Nishio A, Khosla VK. Extravasation of contrast medium from the lenticulostriate artery following local intracarotid fibrinolysis. Surg Neurol. 1993. 39:315–319.
8. Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004. 35:876–881.
9. Yokogami K, Nakano S, Ohta H, Goya T, Wakisaka S. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery. 1996. 39:1102–1107.
10. del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998. 65:1–9.
11. del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003. 23:879–894.
12. Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke. 2002. 33:2100–2104.
13. Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004. 363:768–774.
14. Berger C, Fiorelli M, Steiner T, Schabitz WR, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001. 32:1330–1335.
15. Adams HP Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003. 34:1056–1083.
16. Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003. 34:2025–2030.
17. Broderick JP, Hacke W. Treatment of acute ischemic stroke: Part II: neuroprotection and medical management. Circulation. 2002. 106:1736–1740.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download