This article has been
cited by other articles in ScienceCentral.
Abstract
Helical CT angiography has been widely used in both pre- and post-aortic stent grafting and it has been confirmed to be the preferred modality when compared to conventional angiography. The recent development of multislice CT (MSCT) has further enhanced the applications of CT angiography for aortic stent grafting. One of the advantages of MSCT angiography over conventional angiography is that the 3D reconstructions, based on the volumetric CT data, provide additional information during follow-up of aortic stent grafting. While endovascular repair has been increasingly used in clinical practice, the use of 3D MSCT imaging in endovascular repair continues to play an important role. In this pictorial essay, we aimed to discuss the diagnostic performance of 3D MSCT angiography in post aortic stent grafting, including the most commonly used surface shaded display, curvilinear reformation, the maximum intensity projection, volume rendering and virtual endoscopy. The advantages and disadvantages of each 3D reconstruction are also explored.
Go to :

Keywords: Aneurysm, abdominal, Computed tomography (CT), Stents and prostheses, Images, processing
Since its first introduction in clinical practice more than a decade ago, endovascular repair of abdominal aortic aneurysm (AAA) has been widely performed and it is reported to be an effective alternative to conventional open surgery, especially for the patients with comorbid medical conditions (
1-
3). Unlike open surgical repair of AAA, successful completion of endovascular repair largely depends on the medical imaging, and helical CT angiography (CTA) has been confirmed to be the preferred modality for both the preoperative planning and the postoperative follow-up of endovascular aortic repair (
4,
5).
The development of multislice CT (MSCT) has provided important advantages over single slice CT with regard to CT angiography of the aorta and aortic stent grafting (
6,
7). MSCT enables faster scans than single slice CT by providing high-volume coverage and thin-section images within a single breath hold, and this results in improved spatial resolution in the longitudinal plane. MSCT has been reported to be superior to single slice CT for nearly all clinical applications (
6). Helical CTA for imaging the aorta and stent grafting has been complemented by a series of 3D postprocessing reconstructions, including the surface shaded display (SSD), maximum-intensity projection (MIP), curvilinear reformation (CVR), volume rendering (VR) and virtual intravascular endoscopy (VIE). These 3D reconstructions have been applied for both the pre-and postoperative assessment of aortic stent grafting. However, the 2D axial CT images still remain the standard reference in most of the pre-operative situations such as measurements of the aneurysm sac and neck diameter, visualization of aortic wall calcification and etc (
8). In contrast, the 3D CT images were found to be useful for visualization of the relationship between the stent graft and the arterial branches (
9-
11). Assessing the diagnostic performance of these 3D reconstructions will assist clinicians to choose the appropriate image visualization for assessing endovascular aortic repair, and also to make efficient use of the MSCT imaging modality for clinical purposes. In this pictorial essay, we discussed the diagnostic value of each of the 3D reconstructions that were based on MSCT angiography scanning for a group of patients with AAAs that were treated with aortic stent grafting.
Patient Data and Multislice CT Scanning Protocol
The study was performed on 18 patients (15 men and 3 women, mean age: 75, age range: 63-84) who underwent endovascular repair of abdominal aortic aneurysms between 1999 and 2001. The patients were recommended to receive stent graft treatment from the vascular surgeons because they were unsuitable for open surgical repair due to comorbid medical conditions (hypertension and cardiovascular disease). All the patients were treated with the Zenith AAA Endovascular Graft (William Cook Europe, Bjaeverskov, Denmark) with a suprarenal uncovered component placed above the renal arteries for obtaining proximal fixation. The stent graft is an endoprosthesis constructed of nitinol monofilament that's woven into a tubular zigzag configuration. A thin woven polyester fabric was used to cover the nitinol wire frame. The patients were referred for follow-up examinations at one week, one month, three months, six months and one year after endovascular, and then annually. The initial CT scan was performed on a single slice CT scanner, and the most recent model that was used was on a multislice CT scanner. Therefore, only MSCT data were used for the 3D reconstructions. MSCT angiography was performed on a 16-slice scanner (Toshiba Medical Systems Europe, Netherlands) with the following scanning protocol: 16×0.75 mm beam collimation, a pitch of 2.0 and a reconstruction interval of 1 mm. MSCT angiography was performed with an intravenous injection of 100 ml of non-ionic contrast media (Niopam 300, Bracco UK Ltd. High Wycombe) administered at a rate of 2 ml/second with a scan delay of 30 seconds.
Go to :

3D Image Generation
The CT volume data were converted from the original DICOM (Digital Imaging and Communication in Medicine) images with using commercially available software Analyze V 5.0 (
www.Analyzedirect.com Mayo Clinic, USA). Bony structures were removed by using the region of interest drawings for generating a series of SSD and MIP images. The connectivity and opacity transfer function for the VR were determined so as to maximize the vascular visualization while minimizing the non-vascular display. Visualization of VIE images was based on CT number thresholding, and the details of generating the VIE images have been described elsewhere (
9). The CVR was generated by setting the line at the centre of the abdominal aorta in order to produce coronal or sagittal images for demonstrating the stent graft and the aortic branches. Technical details for the generation of these 3D reconstructions have been described elsewhere (
12).
Go to :

3D Visualization and the Diagnostic Performance of Multislice CT
3D reconstructions were successfully generated for all the patients. The follow-up time ranged from 24 months to 54 months, with a mean period of 40 + 7.6 months. Endoleaks were found in five cases, with type I and III in one patient each, respectively, and type II in three cases. The renal function was evaluated by measuring the serum creatinine levels and it was not significantly affected in all cases, except in one patient who developed chronic renal failure due to an atrophic left renal artery and that patient received renal dialysis. Distal stent graft migration occurred in four patients and their treatment was under observation at the current follow-up.
Surface Shaded Display (SSD)
The SSD represents the surface of a structure within a volume dataset. It is quickly generated because it relies on simple thresholding. It has superiority for the speed and flexibility of rendering images for the visualization of the stent graft and the arterial branches (
Fig. 1A). However, the surface of the is derived from only a small proportion of the available data (less than 10%), and it is prone to artifacts (
Fig. 2A), and this is especially apparent in the patients treated with stent graft due to the high density of the metal wires. Thus, calcification in the aortic wall and the configuration of the aneurysm sac cannot be accurately assessed on the SSD images when compared to the corresponding MIP images (
Figs. 1B,
2B). SSD has only a small role to play in the follow-up of aortic stent grafting as no additional information can be obtained from it when compared to the axial 2D images (
8).
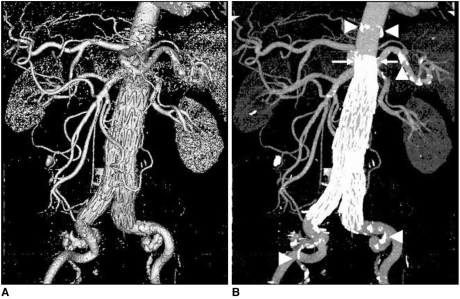 | Fig. 1An 84-year-old man with an infrarenal abdominal aortic aneurysm was treated with an aortic stent graft and he was followed-up at 48 months. The multislice CT surface shaded display clearly shows the aortic branches and the stent graft. The corresponding multislice CT maximum-intensity projection image clearly demonstrates the calcification in the aortic wall besides the aortic stent graft, and the arterial branches. The suprarenal stent grafts are shown deployed above the renal arteries (arrows). Arrowheads point to the calcification. (Reprint with permission from Ref 13) 
|
 | Fig. 2
A. Surface shaded display image in an 80-year-old man shows abdominal aortic aneurysm after endovascular repair. Although the aortic branches can be visualised, calcifications in the aortic wall cannot be clearly demonstrated when compared to the corresponding maximum-intensity projection image as shown in figure 2B (arrows). 
|
Maximum Intensity Projection (MIP)
The MIP was found to be valuable for post stent grafting as it creates angiographic-like images that are similar to those from conventional angiographic examinations. High density calcification, contrast-enhanced vessels, aortic stent wires, the 3D relationship of the aortic stent graft and the arterial branches can be clearly visualized on the MIP images (
Figs. 1B,
2B). The MIP images were also found to be useful for assessment of stent graft migration at the regular follow-ups, and they were reported to be more accurate than the axial 2D images (
Fig. 3) (
13). One of the disadvantages of MIP generation is having to remove the bony component from the volume data, which is a time-consuming procedure. However, development of computer software allows the user to automatically remove bony structures and the processing time has been significantly decreased, which makes MIP image processing an acceptable technique in routine clinical practice.
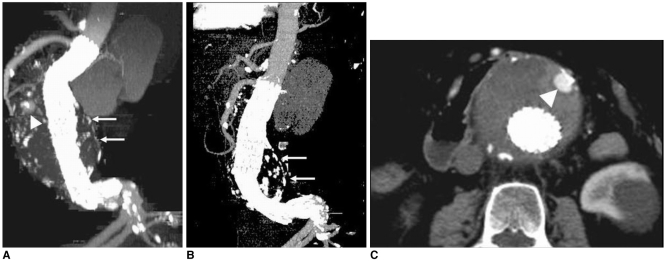 | Fig. 3A 74-year-old man was found to have distal stent migration of 10.2 mm due to foreshortening of the longitudinal aneurysm sac at a
24-month follow-up period ( A, B). Arrows in A and B indicate the aneurysm sac, while arrowheads in A and C point to a type II endoleak due to retrograde flow of the patent inferior mesenteric artery, which resolved spontaneously.(Reprint with permission from Ref 13) 
|
Curvilinear Reformation (CVR)
Most of the abdominal aortic aneurysms are angulated to a variable extent and they have a curved path along the abdominal aorta, so conventional multiplanar reformation is not always able to reveal the anatomical information required for assessment. Curvilinear reformation allows for generating images with the desired anatomy by segmenting the anatomical structure with a curved central lumen line (
Fig. 4). However, caution should be taken into account when choosing the selection plane as the line must be set in the centre of anatomical structures, otherwise, not all of the information will be displayed in the reformatted images and some of the information will be missed (
Fig. 5). Therefore, a series of CVR images instead of individual ones are required for accurately evaluating aortic stent grafting, which is the main limitation of the CVR.
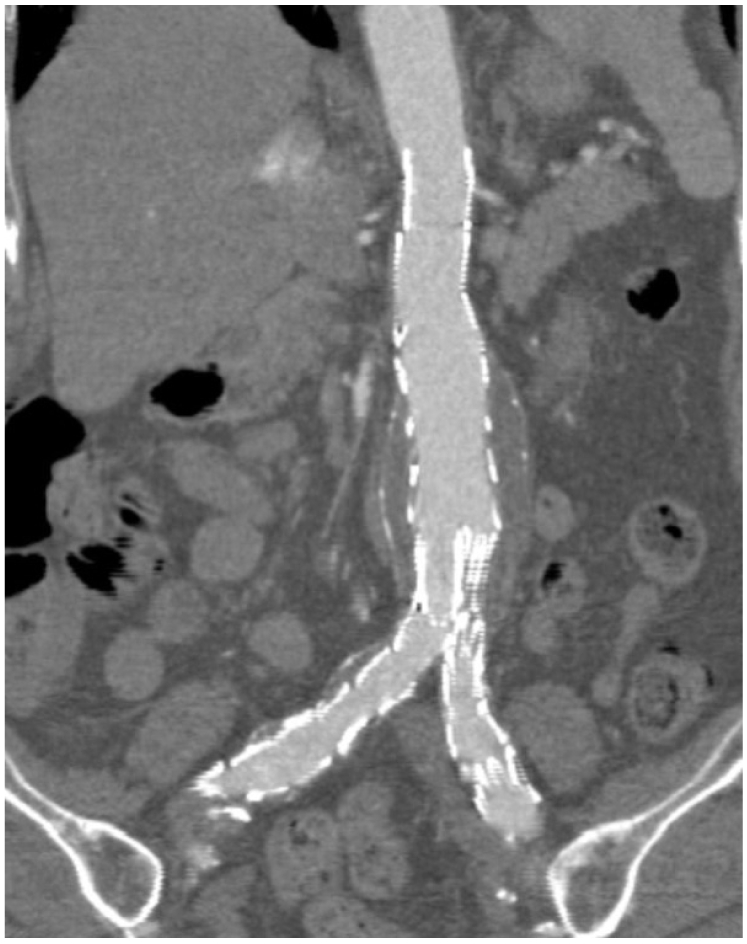 | Fig. 4Curvilinear reformation reconstructions in a patient following endovascular repair of abdominal aortic aneurysm. The aortic branches, the stent graft and the aneurysm sac are shown altogether in the figure. 
|
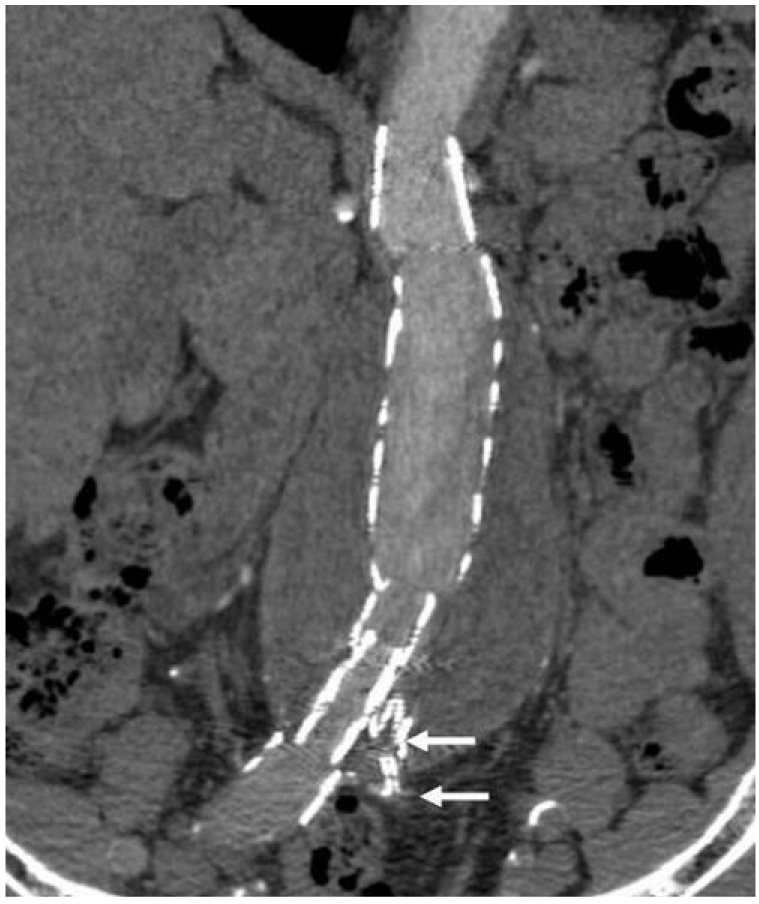 | Fig. 5Curvilinear reformation reconstruction in another patient after endovascular repair of abdominal aortic aneurysm. In contrast to what is shown in figure 4, not all of the information is displayed in this image and the left common iliac artery is missing (arrows) as this anatomy is not in the selected plane. 
|
Volume Rendering (VR)
VR was able to demonstrate all the anatomical structures such as the stent graft and aortic branches because it uses all of the information contained inside a volume dataset. Moreover, each anatomical structure can be coded with a specific colour and opacity value for every attenuation value of the CT data (
Fig. 6A). This allows observers to easily identify a particular structure within the volume dataset and it clearly demonstrates the 3D relationship between the stent graft and the arterial branches (
Figs. 6B, C ). Because the generation of VR images relies on the threshold selection and there is no requirement to remove unwanted structures, the processing time is very short, i.e., only a few minutes (
14). The main limitation of VR is that the final image quality depends on the segmentation of the volume dataset that is used by the rendering algorithm (
Fig. 7). Therefore, acquisition of the optimal source of CT data is of utmost importance to ensure the VR image quality.
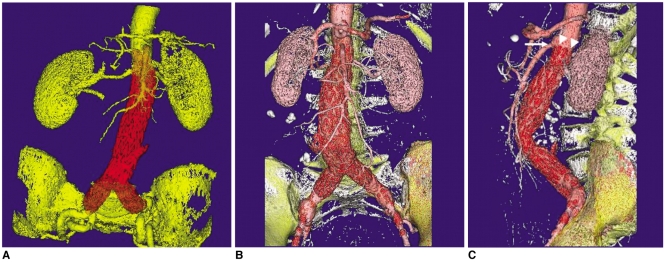 | Fig. 63D volume rendering images were generated in a 65-year-old woman at 36 months after endovascular repair. Different colours were applied to various structures, including the aortic stent graft, arteries and bones (A-C). As shown in the sagittal view (C), the top of the suprarenal stent (arrowheads) was placed just below the superior mesenteric (arrow) artery. 
|
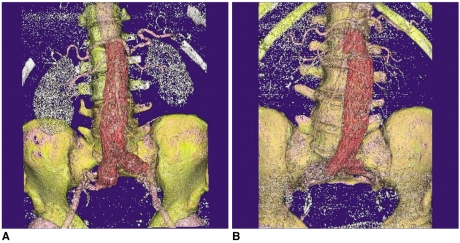 | Fig. 73D volume rendering images were acquired in two patients with suboptimal source image quality, which affects the visualization of the anatomical structures on volume rendering. The signal to noise ratio measured in these two cases was less than 10 (6-8.2) at the levels of the renal artery and the common iliac arteries, which resulted in poor demonstration of the renal parenchyma or the common iliac arteries (A, B). 
|
Virtual Intravascular Endoscopy (VIE)
Unlike other 3D reconstructions, VIE provides unique intraluminal information about the stent wires relative to the arterial branch ostia. The number of stent wires crossing the arterial ostia and the configuration of encroachment can be clearly visualized on VIE (
Fig. 8). The VIE images can enhance our understanding of the effect of the aortic stent graft on the arterial branch ostia, especially in the patients with AAA that is treated with a suprarenal stent graft (
8,
9). Our experience has shown that VIE could be a valuable visualization technique for the follow-up of aortic stent grafting. Comparison of the VIE images at regular follow-up also allows clinicians to assess the effect of stent wires on the arterial ostia, or we can observe the stent wires regarding the distal migration or the reduction of the cross-sectional area of the aortic ostia (
Fig. 9) (
13). One of the limitations of VIE visualization is that it is a time-consuming procedure, which takes about 30 minutes. However, with acquiring more experience, the time required for generating VIE can been greatly reduced. Another limitation is that the stent wire thickness is overestimated on VIE images due to the point spread function, which makes a stent wire looks thicker that it really is (
Fig. 9). Thus, the actual coverage of the stent wires to the aortic ostia is hard to estimate, although we know that only a small proportion of the aortic ostium is covered (
15).
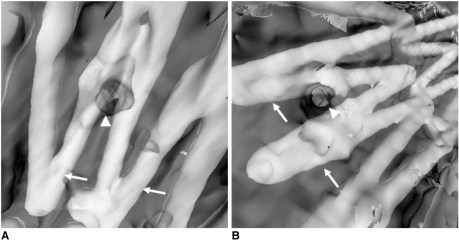 | Fig. 8Virtual intravascular endoscopy images acquired from two patients after endovascular repair of abdominal aortic aneurysms shows the different encroachment of stent wires to the renal ostia. Two stent wires were shown to cross the left renal ostium peripherally in A, while one stent wire crosses the right renal ostium peripherally as shown in B. Arrows indicate the stent wires, while arrowheads point to the renal ostia. 
|
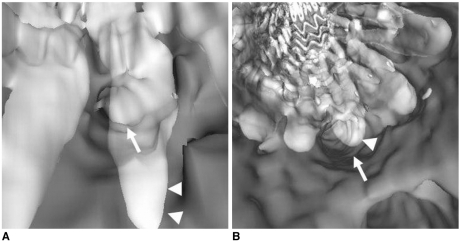 | Fig. 9An 81-year-old woman with an abdominal aortic aneurysm was treated with a suprarenal stent graft and she was followed-up at 36 months. A 10.2 mm stent migration was noted in the most recent CT maximum-intensity projection image ( B) when compared to the previous one ( A). Virtual intravascular endoscopy shows that the superior mesenteric artery ostium was encroached by stent wire, but its position of encroachment shifted due to the movement of stent wires, which was caused by the longitudinal foreshortening of the aneurysm sac. The superior mesenteric artery ostium seems to be covered more than 50% by the stent wire due to the overestimated thickness of wire diameter. The arrow denotes the superior mesenteric artery, while arrowheads refer to the stent wires. (Reprint with permission from Ref 13) 
|
Go to :

DISCUSSION and CONCLUSION
With the introduction of MSCT scanners, CT angiography of the aortic stent graft is becoming a more important procedure. In contrast to single slice CT, CTA can be performed more efficiently with MSCT scanners because of the faster scanning speed and the higher spatial and temporal resolution. In an early study, Rubin et al. (
7) showed that performing CTA with a four-detector row CT scanner was faster, and the scanning was possible with thinner collimation and a reduced dose of contrast medium. However, the studies using 16 slice CT scanners with a submillimeter section thickness demonstrated higher quality CT angiograms than did those obtained from 4 slice scanners for the evaluation of aortoiliac and lower extremity arteries (
16,
17). With the recent introduction of the 64 slice scanner, spiral scanning with even a shorter time represents a further leap in improving the spatial and temporal resolution for routine clinical applications of CTA (
18,
19). Therefore, for the patients undergoing stent graft treatment, CT angiographic examinations with submillimeter resolution in the pure arterial phase will become feasible even for an extended anatomic range. Two of our patients' volume source data were suboptimal and the image quality of VR was affected, as is shown in
Figure 7. For one patient, the CT attenuation was measured to be lower than 200 HU at the level of the renal artery. Although adequate contrast enhancement was obtained in another patient (CT attenuation of 230 HU), the image quality was still affected due to the low signal to noise ratio (increased image noise). We believe that the improved longitudinal resolution with a 64 slice scanner will overcome these limitations that we encountered in our study.
3D CT postprocessing and reconstruction has become a part of the clinical protocol and this has been widely used as an effective alternative to conventional angiography for preoperative planning and the post-operative follow-up of aortic stent grafts (
5,
20). 3D CT angiography is our routine imaging modality to obtain the essential measurements for planning aortic stent graft placement, to assess the patency of stent-covered renal arteries and to detect endoleaks for post stent grafting follow-up (
8). Our results have demonstrated the potential value of 3D MSCT reconstructions, including MIP, VR and VIE, which provide clinicians with additional information for evaluating the effects of endovascular repair. MIP clearly shows the aortic stents and the arterial branches, and it was more accurate for measuring the stent graft migration than were the conventional 2D images (
13), while VR is an efficient visualization tool for demonstrating the 3D relationship between the stent graft and the arterial branches. As the long-term effects of aortic stent grafting are unclear, and especially the effects of transrenal fixation, the additional information provided by VIE is considered valuable for surgeons to assess the outcomes of transrenal fixation in the following two settings: potential interference with renal blood flow by demonstrating the encroachment of stent wires to the renal artery ostia, which may alter the haemodynamics and lead to the dispersion of late multiple emboli (
21), or any reduction of cross-sectional area of the aortic branch ostia that's caused by the presence of stent wires (
15), which may affect the physiologic change of renal function such as renal perfusion. The diagnostic value of CT VIE in aortic stent grafting has been discussed in detail elsewhere (
8,
9,
13,
15).
In conclusion, based on our experience, 3D CT reconstructions offer additional information when compared to the 2D axial images in post-stent grafting. Reliable recognition of the diagnostic performance of each reconstruction is of paramount importance for clinicians to effectively utilize the MSCT imaging technique as some of the 3D postprocessings are time-consuming such as occurs with MIP and VIE. VR is the most efficient reconstruction method for visualizing the aortic stent graft relative to the arterial branches, whereas VIE is a valuable tool for visualizing intraluminal encroachment of the stent wires to the aortic ostia.
Go to :

Acknowledgments
The author would like to thank Dr. Ellis, Dr. Collins and Dr. Kennedy for their expertise and clinical cooperation.
Go to :

References
1. Buth J, van Marrewijk CJ, Harris PL, Hop WC, Riambau V, Laheij RJF, et al. Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: a report on the EUROSTAR experience. J Vasc Surg. 2002; 35:211–221. PMID:
11854717.

2. Cao P, Verzini F, Parlani G, Romano L, De Rango P, Pagliuca V, et al. Clinical effect of abdominal aortic aneurysm endografting: 7-year concurrent comparison with open repair. J Vasc Surg. 2004; 40:841–848. PMID:
15557895.

3. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004; 351:1607–1618. PMID:
15483279.

4. Broeders IA, Blankensteijn JD, Olree M, Mali W, Eikelboom BC. Preoperative sizing of grafts for transfemoral endovascular aneurysm management: a prospective comparative study of spiral CT angiography, arteriography and conventional CT imaging. J Endovasc Surg. 1997; 4:252–261. PMID:
9291050.

5. Armerding MD, Rubin GD, Beaulieu CF, Slonim SM, Olcott EW, Samuels SL, et al. Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. Radiology. 2000; 215:138–146. PMID:
10751479.

6. Hu H, He HD, Foley WD, Fox SH. Four multidetector-row helical CT: image quality and volume coverage speed. Radiology. 2000; 215:55–62. PMID:
10751468.

7. Rubin GD, Shiau MC, Leung AN, Kee ST, Logan LJ, Sofilos MC. Aorta and iliac arteries: single versus multiple detector-row helical CT angiography. Radiology. 2000; 215:670–676. PMID:
10831682.

8. Sun Z, Winder RJ, Kelly BE, Ellis PK, Kennedy PT, Hirst DG. Diagnostic value of CT virtual intravascular endoscopy in aortic stent grafting. J Endovasc Ther. 2004; 11:13–25. PMID:
14748633.
9. Sun Z, Winder RJ, Kelly BE, Ellis PK, Hirst DG. CT virtual intravascular endoscopy of abdominal aortic aneurysms treated with suprarenal endovascular stent grafting. Abdom Imaging. 2003; 28:580–587. PMID:
14580104.

10. Davis CP, Ladd ME, Romanowski BJ, Wildermuth S, Knoplioch JF, Debatin JF. Human aorta: preliminary results with virtual endoscopy based on three-dimensional MR imaging data sets. Radiology. 1996; 199:37–40. PMID:
8633169.

11. Neri E, Bonanomi C, Vignali R, Cioni R, Ferrari M, Petruzzi P, et al. Spiral CT virtual endoscopy of abdominal arteries: clinical applications. Abdom Imaging. 2000; 25:59–61. PMID:
10652924.

12. Luccichenti G, Cademartiri F, Pezzella FR, Runza G, Belgrano M, Midiri M, et al. 3D reconstruction techniques made easy: know-how and pictures. Eur Radiol. 2005; 15:2146–2156. PMID:
15809826.

13. Sun Z. Three-dimensional visualization of suprarenal aortic stent-grafts: evaluation of migration in midterm follow-up. J Endovasc Ther. 2006; 13:85–93. PMID:
16445328.
14. Sun Z, Zheng H. Helical CT angiography of aortic stent grafting: Comparison of three-dimensional rendering techniques. Lecture Notes in Computer Sciences. 2004; 3314:544–549.

15. Sun Z, Zheng H. Cross-sectional area reduction of the aortic ostium by suprarenal stent wires: in vitro phantom study by CT virtual angioscopy. Comput Med Imaging Graph. 2004; 28:345–351. PMID:
15294312.

16. Willmann JK, Baumert B, Schertler T, Wildermuth S, Pfammatter T, Verdun FR, et al. Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital subtraction angiography. Radiology. 2005; 236:1083–1093. PMID:
16055691.

17. Schertler T, Wildermuth A, Alkadhi H, Kruppa M, Marincek B, Boehm T. Sixteen-detector row CT angiography for lower-leg arterial occlusive disease: analysis of section width. Radiology. 2005; 237:649–656. PMID:
16244274.

18. Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005; 46:552–557. PMID:
16053973.

19. Flohr T, Stierstorfer K, Raupach R, Ulzheimer S, Bruder H. Performance evaluation of a 64-slice CT system with z-flying focal spot. Rofo. 2004; 176:1803–1810. PMID:
15573292.

20. Rydberg J, Kopecky KK, Lalka SG, Johnson MS, Dalsing MC, Persohn SA. Stent grafting of abdominal aortic aneurysms: pre- and postoperative evaluation with multislice helical CT. J Comput Assist Tomogr. 2001; 25:580–586. PMID:
11473190.
21. Chong CK, How TV. Flow patterns in an endovascular stent-graft for abdominal aortic aneurysm repair. J Biomech. 2004; 37:89–97. PMID:
14672572.

Go to :














 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download