Abstract
Objective
We wanted to compare the transaxial source images with the optimized three plane, thin-slab maximum intensity projection (MIP) images from electrocardiographic (ECG)-gated cardiac CT for their ability to detect hemodynamically significant stenosis (HSS), and we did this by means of performing a receiver operating characteristic (ROC) analysis.
Materials and Methods
Twenty-eight patients with a heart rate less than 66 beats per minute and who were undergoing both retrospective ECG-gated cardiac CT and conventional coronary angiography were included in this study. The contrast-enhanced CT scans were obtained with a collimation of 16×0.75-mm and a rotation time of 420 msec. The transaxial images were reconstructed at the mid-diastolic phase with a 1-mm slice thickness and a 0.5-mm increment. Using the transaxial images, the slab MIP images were created with a 4-mm thickness and a 2-mm increment, and they covered the entire heart in the horizontal long axis (4 chamber view), in the vertical long axis (2 chamber view) and in the short axis. The transaxial images and MIP images were independently evaluated for their ability to detect HSS. Conventional coronary angiograms of the same study group served as the standard of reference. Four radiologists were requested to rank each image with using a five-point scale (1 = definitely negative, 2 = probably negative, 3 = indeterminate, 4 = probably positive, and 5 = definitely positive) for the presence of HSS; the data were then interpreted using ROC analysis.
Results
There was no statistical difference in the area under the ROC curve between transaxial images and MIP images for the detection of HSS (0.8375 and 0.8708, respectively; p > 0.05). The mean reading time for the transaxial source images and the MIP images was 116 and 126.5 minutes, respectively.
With the arrival of multi-detector row technology that's combined together with subsecond rotation and retrospective electrocardiographic (ECG) gating, computed tomography (CT) has become a clinically important, noninvasive diagnostic technique for performing cardiac imaging (1-5). Multi-detector row CT (MDCT) has proven to be a promising technique for detecting high-grade coronary artery stenosis and coronary artery occlusion, and this technique has a sensitivity of 73-86% and a specificity of 93-98% (3, 4, 6-8). CT angiography has been evolving rapidly, yet visualizing the coronary arteries with sufficient image quality and an adequate postprocessing technique still remains as a challenge (9-13). The transaxial images of the coronary arteries are difficult to interpret because of the tortuous course of these vessels, and so several reconstruction techniques such as multiplanar reconstruction (MPR), maximum intensity projection (MIP), volume rendering technique (VRT) and shaded surface display (SSD) have been developed for evaluating 3D MR or CT angiography data sets (6, 8, 14-17). However, the 3D rendering techniques require manual input prior to reconstruction, such as segmentation, image cutting and thresholding, and this input can be time-consuming and the process is prone to error (16, 17). In contrast, MIP reconstructions do not necessarily require thresholding or image cutting, and they can be done quickly. Moreover, the MIP algorithm is now widely available, in the same form as it is employed on the most commonly used workstations. Various 2D and 3D techniques have been for the postprocessing of CT angiography of the other organs, and several studies have investigated their diagnostic accuracy as compared with the evaluation of the transaxial images (12, 18, 19). To date, with respect to coronary artery imaging, the comparison between MIP and source images has been done with using MR coronary angiography (20), and only one study has reported that the source images were more accurate than the other visualization techniques for detecting coronary artery stenosis on MDCT (6). To the best of our knowledge, there have been no reports that have focused on the issue of image data volume and that have compared the relative diagnostic values of "thin-slab MIP" to those of the original transaxial images for detecting hemodynamically significant stenosis (HSS) of the coronary arteries. Therefore, the goal of this study is to compare, by means of receiver operating characteristic (ROC) analysis, the original transaxial images with the technically optimized "3-plane, thin-slab MIP" images to determine their usefulness for detecting HSS.
Our hospital's institutional review board approved the research protocols, and all patients gave us their informed written consents.
We retrospectively studied 42 patients who had consecutively undergone MDCT as well as having undergone invasive coronary angiography over a 4-month period. All the examinations were done for the patients with signs of ischemic heart disease. For the patients with a heart rate greater than 65 bpm, 100 mg of the beta-blocker (Betaloc® [metoprolol tartrate]; Yuhan Corporation, Seoul, Korea) was administered one hour prior to the CT examination. If after the administration of the beta-blocker the patients still had heart rates higher than 66 bpm during the CT scan, then they were excluded from this study. Finally, 28 patients comprised our study group. There were 19 men and nine women (age range: 40-72 years, mean age: 56.2 years). The average time between the two examinations was 1.8 days (range: 0-14 days). The mean patient heart rate was 54.7 bpm (range: 31-65.0 bpm).
Coronary angiography was performed with different technical systems and with using Judkin's technique. At least four views of the left coronary arterial (LCA) system and two views of the right coronary arterial (RCA) system were obtained and stored on a CD-ROM. One cardiac radiologist (J.B.S.) evaluated the angiogram for the presence of HSS by performing quantitative analysis of the coronary angiograms, and the locations of the lesions were systemically recorded. The result served as the standard of reference. Coronary arteries 1.5 mm or greater in size were included in the analysis, and 175 coronary arteries of 28 patients were interpreted. There were 28 left main coronary arteries, 28 left anterior descending arteries, 27 left circumflex coronary arteries, 28 right coronary arteries, 26 diagonal branches, three ramus intermedius, 21 obtuse marginal arteries and 14 posterior descending arteries. HSS was defined as a degree of stenosis 50% or greater than the expected internal luminal diameter of the coronary artery; a degree of stenosis less than 50% was considered to be negative for HSS. Among the coronary arteries that we analyzed, there were 127 (73%) normal coronary arteries and 48 (27%) coronary arteries with HSS (Table 1).
Each patient's heart rate (HR) was measured prior to the examination. The patients with a pre-scan HR ≥ 65 bpm were given 100 mg of metoprolol per os one hour before the scan. The scanning parameters for the MDCT coronary angiography (Sensation 16; Siemens, Forchheim, Germany) were: 12 detectors (because the retrospectively electrocardiographically gated protocol did not allow the use of all 16 rows), individual detector width: 0.75 mm, gantry rotation time: 420 ms, 120 kVp, 500 mAs, feed/rotation 2.8 mm, and scanning was done in the cranio-caudal direction. 100 ml of contrast agent (Ultravist370®; Schering, Berlin, Germany) were injected intravenously in a rate of 4 mL/s, and this was followed by 50 ml of saline at the same rate. Early scanning started with real-time bolus tracking (CARE bolus; Siemens, Forchheim, Germany) using an ROI in the ascending aorta for monitoring a threshold of +100 HU above the baseline attenuation.
The images were reconstructed retrospectively with a slice thickness of 1 mm and an interval of 0.5 mm with using a medium smooth kernel (B30f). The position of the reconstruction window within the cardiac cycle was individually optimized to minimize the motion artifacts.
The post-processing was performed on a commercially available workstation (Wizard; Siemens) and all of it was done by one radiologist (J.B.S.). We created three planes of parallel 4-mm, thin-slab, MIP images (Figs. 1, 2) at 2-mm increments to cover the entire heart in the horizontal long axis (4 chamber view) from the superior surface to the diaphragmatic surface of the left ventricle, in the vertical long axis (2 chamber view) from the left ventricle to the right ventricle, and in the short axis of the heart across the atrioventricular groove (10). All the transaxial and MIP images were displayed on a 512×512 matrix. All the images were sent to a picture archiving and communication system (PACS).
Our study consisted of three sessions: 1) teaching and pre-interpretation, 2) interpretation of transaxial images, and 3) interpretation of the thin-slab MIP images. In the teaching and pre-interpretation session, the task was explained to each observer. We emphatically emphasized that as only a degree of stenosis of 50% or greater was considered to be hemodynamically significant, a degree of stenosis less than 50%, e.g. 30% or 45%, had to be scored as Grade 1 (normal). Each observer had a pre-interpretation session that simulated real interpretation with employing three patients who were not included in this study. After that session, the observers were questioned whether or not they correctly understood the definition of hemodynamically significant stenosis. Any uncertainty was thoroughly discussed with the radiologist who designed this study (J.B.S.). The next session included interpretation of the transaxial or MIP images. The images in each set were randomly ordered. All the image interpretations were performed on a PACS monitor by four Board-certified radiologists (S.I.C., K-H.D., S.M.K. and W.L.); they were all accustomed to using the PACS viewer. Each radiologist was blinded to the patient histories and the results of their conventional coronary angiograms; during each session the radiologists worked independently and they were separated from each other. Two readers first interpreted the transaxial images while the other two readers first interpreted the MIP images. To diminish the learning effects, a minimum of one week was allowed to elapse between each reading session. To simulate the routine clinical interpretation environment, the readers were allowed to interactively adjust the brightness and contrast of the images. According to reader's preference, they used a ruler from the PACS tool or they examined the images with the naked eye to evaluate HSS without the aid of PACS tools. The use of other tools such as magnification and contrast adjusting was also allowed. All digital operations were performed using a computer mouse. As is done in conventional coronary angiography, the left circumflex coronary arteries, right coronary arteries, diagonal branch, ramus intermedius, obtuse marginal arteries and posterior descending arteries having a diameter of 1.5 mm or greater were evaluated. A degree of stenosis ≥ 50% was considered to represent HSS. For the ROC curves, the likelihood of the presence of significant stenosis was assessed by using the following five-grade scoring system; 1 = definitely negative, 2 = probably negative, 3 = indeterminate, 4 = probably positive, and 5 = definitely positive. These responses were recorded and re-sorted by each system for the statistical analysis. The time required for each observer to interpret with using each method was also recorded.
The observer performance for the detection of HSS of the coronary artery with using the two visualization techniques was determined by means of ROC analysis of the individual and averaged reader data. To allow for generalization to our article readers and their cases, we used a multireader-multicase ROC approach (LABMRMC; Chicago University, Charles, E. Metz). The statistical significance of the results for each technique was reported as a 95% confidence interval (CI) for the mean difference in the area under the ROC curve (Az value) for the observer performance. Mean differences were regarded as being statistically significant at the 5% level when the corresponding CI did not encompass zero. The diagnostic accuracies of the two visualization techniques were evaluated by averaging each sensitivity, specificity and accuracy in two ways; first, when Grade 3 (indeterminate) or higher was regarded as being positive, and second when Grade 4 (probably positive) or higher was regarded as being positive. We compared the differences in the time needed for reading during each session for the transaxial and MIP images with using the Wilcoxon-Signed Ranks test.
The mean Az values, which indicate the performance of each reader, are given in Table 2. The 95% CIs for the mean differences in the Az values between the two image sets and the reading time of each session are also provided. We found that for all three radiologists, the Az values from the MIP images were higher than those Az values from the transaxial images (Fig. 3), however, the difference was significant for only one reader. Overall, the Az values from both image sets were not significantly different. When Grade 3 (indeterminate) or higher were regarded as positive, the sensitivities, specificities, and accuracies were 65%, 86%, and 80% for the transaxial images and 71%, 84%, and 80% for the MIP images, respectively. When Grade 4 (probably positive) was used as the cut-off point, the sensitivities, specificities and accuracies were 47%, 92%, and 80% for the transaxial images and 54%, 92%, and 81% for the MIP images. The mean reading times for the transaxial images and the MIP images were 116 and 126.5 minutes, respectively; these findings were not statistically significant (Wilcoxon-Signed Ranks test, p value = 0.465). The mean numbers of the transaxial and MIP images per patient were 238 and 162, respectively.
To the best of our knowledge, this is the first study to use ROC analysis to compare the usefulness of standardized, 3-plane, thin-slab MIP, which is easily processed in a relatively short time, with the transaxial images in terms of their ability to detect HSS. In this study, no significant difference in the observer performance was noted between their interpretation of the transaxial and the MIP images. Even though it was not statistically significant, the MIP images were slightly superior to the transaxial images for the detection of HSS of the coronary arteries. Although the transaxial images normally represent the clinical reference standard for conducting detailed image analysis, the diagnostic value of the coronary imaging is restricted by the small vascular diameters and the 3-dimensional spreading of the vessels. Consequently, the vascular sections are often displayed at an unfavorable cutting angle or they are affected by the partial volume effect. Therefore, we studied the effective image post-processing modalities for their ability to visualize the coronary arteries, including MPR, MIP, 3D-VRT and virtual endoscopy (VE), which is a derivate of 3D-VRT (6, 8, 16). Vogl et al. (6) have concluded that transverse scanning is the superior technique for evaluating CT coronary angiography. They have also hypothesized that the transaxial imaging revealed the highest sensitivities for detecting atherosclerotic wall changes and it was less susceptible to motion artifacts. Mahnken et al. (8) stated that 3D-VRT is a promising tool for assessing cardiac MDCT data sets, and its sensitivity and specificity for detecting coronary artery stenoses are comparable to those of other visualization techniques. Previous reports have demonstrated that the disadvantages of MIP are that it uses only a fraction of the available data and that the algorithm results in the overestimation of the stenoses (13). Furthermore, many artifacts are known to exist on the MIP images, and no 3D depth can be obtained. The MIP images also have the problem that an eccentric stenosis cannot be detected due to the overlapping artifact. To overcome these problems, we used three planes of parallel, 4-mm, thin-slab MIPs with 2-mm increments. These slabs were moved through the volume, with the slab movement distance smaller than the slab thickness, and we using three planes so that the previously mentioned disadvantages could be overcome. We used three planes of MIP images for evaluating coronary arteries. The underestimation of eccentric stenosis could be avoided because for the interpretation, we used three almost perpendicular imaging planes. In addition, because the imaging planes used in this study were similar to those of cardiac MRI or transthoracic echocardiography, other cardiac abnormalities such as myocardial thinning, chamber enlargement and pericardial effusion could easily be assessed.
The inevitability of reconstruction with sub-millimeter section thickness and increments for the evaluation of coronary artery results in a large number of axial images. Moreover, in those cases with motion artifact, multiple sets of transaxial images should be reconstructed in different cardiac phases in order to identify the optimal cardiac phase for viewing each coronary vessel. This results in a continuous increase in digital data volume, and this is a serious challenge to implement with PACS (21). Although the cost of PACS and the data storage devices is rapidly decreasing, it is important to be able to manage a large amount of data with using the existing systems more effectively, and this is especially important for those clinics and hospitals that cannot afford new systems. As shown in our study, the number of images for each patient can be reduced from 238 to 162 by using the MIP images, and this means there is a smaller digital data volume.
In our study, the mean average reading-time for the transaxial and the MIP images was not significantly different. This is probably because the interpreters might have evaluated the MIP images more carefully and they might have unintentionally noticed that the MIPs would be more useful than the transaxial images for detecting HSS when the task was explained to each observer during the teaching session. Furthermore, not all of our readers are as accustomed to the MIP images as they are to the transaxial images, even though we offered a brief teaching session for interpretation. Finally, because the MIP images consist of three image sets with different image planes, the readers might have used additional time to compare the coronary lesions in the three-plane images. The reconstruction method we used in this study is quite easy and it can be performed by a technician if he/she knows the basic anatomy of the heart. Therefore, we did not regard the time needed for generating the MIP images as additional time spent by the radiologists.
There are several limitations to our study. The first is that those patients with heart rates greater than 66 bpm were excluded. Although a recent study (3) with 16-slice multidetector CT demonstrated an 86% specificity and a 95% sensitivity for the detection of HSS, this result was confined to the patients who had a lower heart rate. Even if we had used a beta-blocker to decrease the heart rate in some patients, it was impossible for some patients to obtain a rate of 65 bpm or less, and so they were excluded from this study. With the advent of the 64-section CT scanner and the rapid gantry rotation technique, more reliable cardiac imaging might be acquired from those patients with higher heart rates, and cardiac arrhythmia and the limitation of a high heart rate may be overcome in the near future. Because the aim of the present study is to compare the usefulness of two techniques in patients with optimally obtained CT, we believe that our strategy is justified. The second limitation is that not all the coronary vessels are included in this study. However, because of the intrinsic limitation in the temporal resolution, even when using a current state-of-the-art CT scanner, most studies on the detection of coronary stenosis have been focused on the evaluation of the coronary artery segments that are larger than 1.5 or 2 mm in diameter. We have attempted to include in our study a greater number coronary artery segments and side branches that had a vessel diameter approximately ≥ 1.5 mm that can be evaluated on conventional coronary angiography, which is a standard of reference. This approach is also practical in terms of the clinical setting because those stenoses in vessels with a diameter < 1.5 mm are rarely included as being targets for revascularization. The third limitation is that we did not use thinner reconstructed 3-plane images to evaluate HSS. If we had been able to afford a larger data storage device or the most up-to-date PACS, we could have reconstructed three plane images with a thinner slice. It could have improved the diagnostic performance of the MIP images for detecting HSS. Lastly, although only 28 patients were included in our study, the 175 segments we evaluated in our study are a sufficient number to perform ROC analysis.
For the clinical purpose of detecting HSS of the coronary arteries, the thin-slab MIP images are comparable to the transaxial images. The digital data volume of the MIP images is significantly lower than that of the transaxial images. Therefore, to evaluate coronary artery stenosis, it is acceptable to use thin slab MIP images without referring to the transaxial images.
Figures and Tables
Fig. 1
Schematic diagram of the range of parallel thin-slab maximum intensity projections arranged in a horizontal long axis (4 chamber view, A), vertical long axis (2 chamber view, B), and short axis of the heart (C).
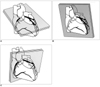
Fig. 2
Representative maximal intensity projection images of the coronary arteries using the standardized 3-plane, thin-slab maximum intensity projection technique. A. Horizontal long axis (4 chamber) view, B. Vertical long axis (2 chamber) view, C. Short axis view.
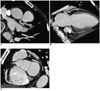
Fig. 3
Receiver operating characteristic curves for the transaxial images and the MIP images from each reader. The Az values of the MIP images were larger than those of the transaxial images except for reader 3. However, the difference was statistically significant only for reader 2. Axial = Transaxial image, TPF = true positive fraction, FPF = false positive fraction.

Table 1
The Number of Normal and Abnormal Coronary Arteries on Conventional Coronary Angiography as a Standard of Reference

Note.-Significant stenosis indicates a vessel with the same or greater than 50% narrowing of the expected internal diameter of the arterial lumen.
LM = left main coronary artery, LAD = left anterior descending coronary artery, LCx = left circumflex coronary artery, RCA = right coronary artery, D = diagonal branch, OM = obtuse marginal branch, RI = ramus intermedius, PDA = posterior descending coronary artery.
Acknowledgements
We thank Bonnie Hami, Department of Radiology, University Hospitals of Cleveland, OH, for her editorial assistance.
References
1. Rumberger JA. Noninvasive coronary angiography using computed tomography: ready to kick it up another notch [editorial]. Circulation. 2002. 106:2036–2038.
2. Nieman K, Oudkerk M, Rensing BJ, van Ooijen P, Munne A, van Geuns RJ, et al. Coronary angiography with multislice computed tomography. Lancet. 2001. 357:599–603.
3. Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation. 2002. 106:2051–2054.
4. Kopp AF, Schroeder S, Kuettner A, Baumbach A, Georg C, Ohnesorge B, et al. Non-invasive coronary angiography with high resolution mutidetector-row computed tomography: results in 102 patients. Eur Heart J. 2002. 23:1714–1725.
5. Kopp AF, Schroeder S, Kuettner A, Heuchmid M, Georg C, Ohnesorge B, et al. Coronary arteries: retrospectively ECG-gated multi-detector row CT angiography with selective optimization of the image reconstruction window. Radiology. 2001. 221:683–688.
6. Vogl TJ, Adolmaali ND, Diebold T, Engelmann K, Ay M, Dogan S, et al. Techniques for the detection of coronary atherosclerosis: multi-detector row CT coronary angiography. Radiology. 2002. 223:212–220.
7. Gerber TC, Kuzo RS, Lane GE, O'Brien PC, Karstaedt N, Morin RL, et al. Image quality in a standardized algorithm for minimally invasive coronary angiography with multislice spiral computed tomography. J Comput Assist Tomogr. 2003. 27:62–69.
8. Mahnken AH, Wildberger JE, Sinha AM, Dedden K, Stanzel S, Hoffmann R, et al. Value of 3D-volume rendering in the assessment of coronary arteries with retrospectively ECG-gated multislice spiral CT. Acta Radiol. 2003. 44:302–309.
9. Addis KA, Hopper KD, Lyriboz TA, Liu Y, Wise SW, Kasales CJ, et al. CT angiography: in vitro comparison of five reconstruction methods. AJR Am J Roentgenol. 2001. 177:1171–1176.
10. Hirai T, Korogi Y, Ono K, Murata Y, Takahashi M, Suginohara K, et al. Maximum stenosis of extracranial internal carotid artery: effect of luminal morphology on stenosis measurement by using CT angiography and conventional DSA. Radiology. 2001. 221:802–807.
11. Rubin G, Dake MD, Napel S, Jeffrey RB, McDonnell CH, Sommer FG, et al. Spiral CT of renal artery stenosis: comparison of three-dimensional rendering techniques. Radiology. 1994. 190:181–189.
12. Berg MH, Manninen HI, Vanninen RL, Vainio RA, Soimakallio S. Assessment of renal artery stenosis with CT angiography: usefulness of multiplanar reformation, quantitative stenosis measurement, and densitometric analysis of renal parenchymal enhancement as adjuncts to MIP film rendering. J Comput Assist Tomogr. 1998. 22:533–540.
13. Heath DG, Soyer PA, Kuszyk BS, Bliss DF, Calhoun PS, Bluemke DA, et al. Three-dimensional spiral CT during arterial portography: comparison of three rendering techniques. RadioGraphics. 1995. 15:1001–1011.
14. Lu Bin, Dai RP, Jiang SL, Bai H, He S, Zhuang N, et al. Effects of window and threshold levels on the accuracy of three-dimensional rendering techniques in coronary artery electron-beam CT angiography. Acad Radiol. 2001. 8:754–761.
15. Rensing BJ, Bongaerts A, van Geuns RJ, van Ooijen P, Oudkerk M, de Feyter PJ. Intravenous coronary angiography using electron beam computed tomography. Prog Cardiovasc Dis. 1999. 42:139–148.
16. Peter MA, van Ooijen P, Ho KY, Dorgelo J, Oudkerk M. Coronary artery imaging with multidetector CT: visualization issues. RadioGraphics. 2003. 08. Available from: URL: http://radiographics.rsnajnls.org/cgi/content/full/e16v1.
17. Pannu HK, Flohr TG, Corl FM, Fishman EK. Current concepts in multi-detector row CT evaluation of the coronary arteries: principles, techniques, and anatomy. RadioGraphics. 2003. 23:s111–s125.
18. Soto JA, Munera F, Cardoso N, Guarin O, Medina S. Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr. 1999. 23:188–196.
19. Sugahara T, Korogi Y, Hirai T, Hamatake S, Komohara Y, Okuda T, et al. CT angiography in vascular intervention for steno-occlusive diseases: role of multiplanar reconstruction and source images. Br J Radiol. 1998. 71:601–611.
20. Regenfus M, Ropers D, Achenback S, Schlundt C, Kessler W, Laub G, et al. Diagnostic value of maximum intensity projections versus source images for assessment of contrast-enhanced three-dimensional breath-hold magnetic resonance coronary angiography. Invest Radiol. 2003. 38:200–206.
21. Bae KT, Whiting BR. CT data storage reduction by means of compressing projection data instead of images: feasibility study. Radiology. 2003. 219:850–855.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download